Abstract
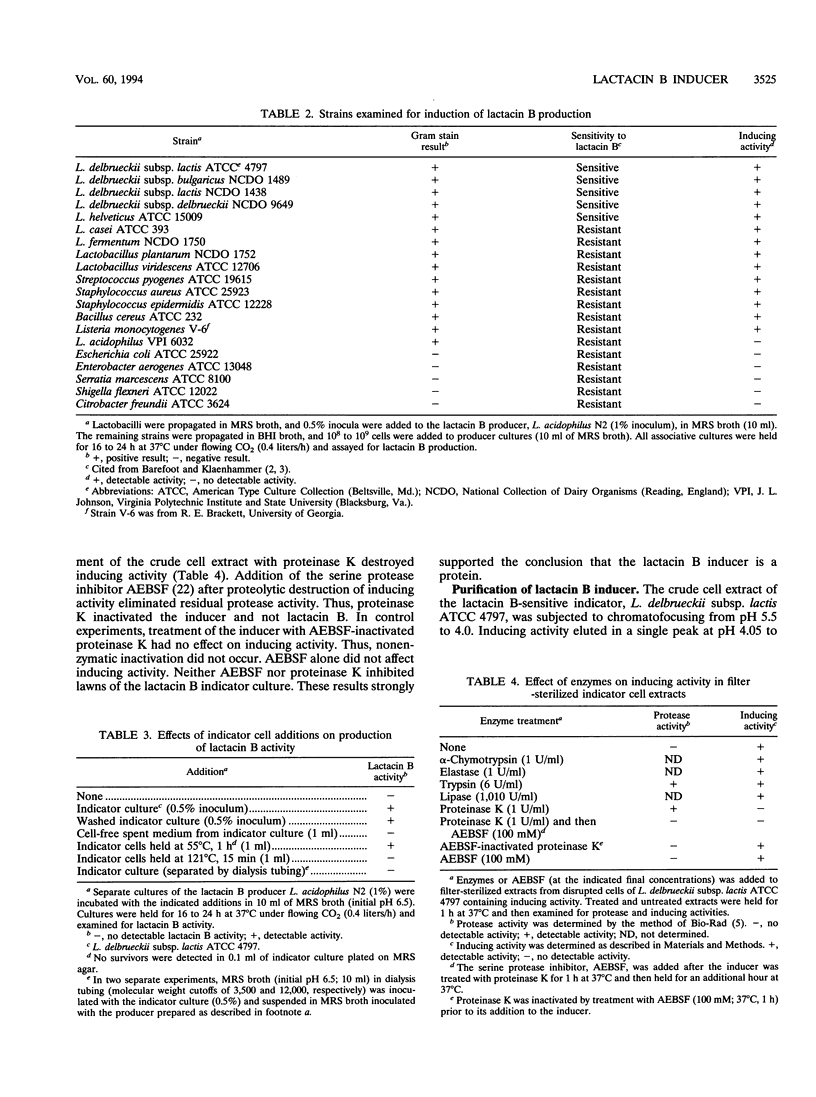
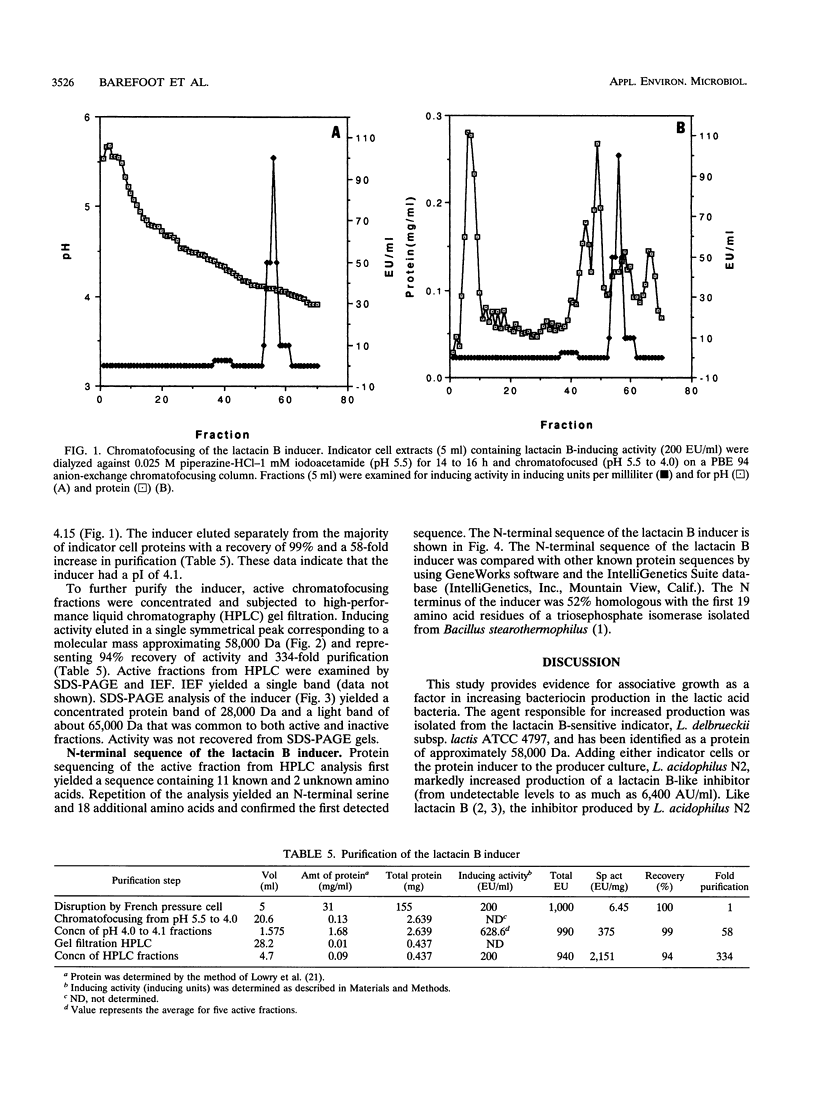
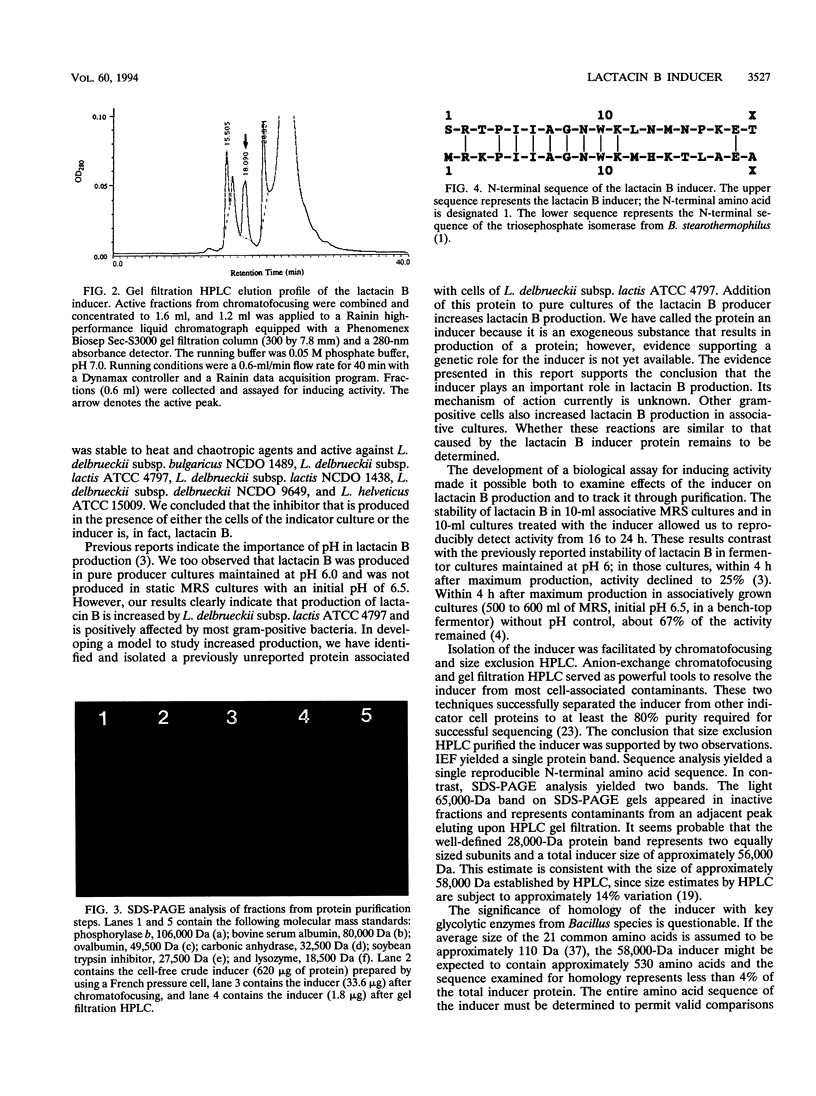
Lactacin B is a heat-stable bacteriocin produced by Lactobacillus acidophilus N2 that is active against closely related lactobacilli, including Lactobacillus delbrueckii subsp. lactis (formerly Lactobacillus leichmannii) ATCC 4797. Pure producer cultures propagated in MRS broth (initial pH 6.5) contain no lactacin B; it is detected only in cultures maintained at pH 5.0 to 6.0 and produced optimally at pH 6.0 S. F. Barefoot and T. R. Klaenhammer, Antimicrob. Agents Chemother. 26:328-334, 1984). Associative growth of producer and indicator, L. delbrueckii subsp. lactis ATCC 4797, resulted in production of an inhibitor identical to lactacin B. Associative growth increased lactacin B production from nondetectable levels (< 100 activity units [AU]/ml) to between 3,200 and 6,400 AU/ml in MRS broth (initial pH 6.5) and resulted in early but equal production of lactacin B (approximately 25,600 AU/ml) in broth maintained at pH 6.0. Indicator cells, but not spent culture filtrates, induced lactacin B production. Indicator cells disrupted by a French pressure cell yielded cell-free filtrates containing inducing activity. Chromatofocusing and gel filtration high-performance liquid chromatography of cell-free filtrates yielded a protein with a pI of 4.1 and a molecular size of approximately 58 kDa that induced lactacin B production. Analytical isoelectric focusing yielded a single protein band. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels contained a 28-kDa protein suggesting a two-subunit structure. Protein sequencing identified an N-terminal serine and 18 additional amino acids. To our knowledge, there are not previous descriptions of proteins that induce bacteriocin production in lactic acid bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artavanis-Tsakonas S., Harris J. I. Primary structure of triosephosphate isomerase from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):599–611. doi: 10.1111/j.1432-1033.1980.tb04755.x. [DOI] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983 Jun;45(6):1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Purification and characterization of the Lactobacillus acidophilus bacteriocin lactacin B. Antimicrob Agents Chemother. 1984 Sep;26(3):328–334. doi: 10.1128/aac.26.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C. L., Gilliland S. E. Bacteriocin involved in premature death of Lactobacillus acidophilus NCFM during growth at pH 6. J Dairy Sci. 1988 Feb;71(2):306–315. doi: 10.3168/jds.S0022-0302(88)79559-4. [DOI] [PubMed] [Google Scholar]
- Héchard Y., Jayat C., Letellier F., Julien R., Cenatiempo Y., Ratinaud M. H. On-line visualization of the competitive behavior of antagonistic bacteria. Appl Environ Microbiol. 1992 Nov;58(11):3784–3786. doi: 10.1128/aem.58.11.3784-3786.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol. 1986 Aug;167(2):439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Cloning, expression, and nucleotide sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin J. J Bacteriol. 1990 Nov;172(11):6339–6347. doi: 10.1128/jb.172.11.6339-6347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993 Sep;12(1-3):39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Cloning, phenotypic expression, and DNA sequence of the gene for lactacin F, an antimicrobial peptide produced by Lactobacillus spp. J Bacteriol. 1991 Mar;173(5):1779–1788. doi: 10.1128/jb.173.5.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987 Mar;53(3):553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl Environ Microbiol. 1991 Jan;57(1):114–121. doi: 10.1128/aem.57.1.114-121.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørtvedt C. I., Nissen-Meyer J., Sletten K., Nes I. F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991 Jun;57(6):1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger U., Kaya M., Lücke F. K. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J Appl Bacteriol. 1991 Jun;70(6):473–478. doi: 10.1111/j.1365-2672.1991.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Sobrino O. J., Rodríguez J. M., Moreira W. L., Cintas L. M., Fernández M. F., Sanz B., Hernández P. E. Sakacin M, a bacteriocin-like substance from Lactobacillus sake 148. Int J Food Microbiol. 1992 Jul;16(3):215–225. doi: 10.1016/0168-1605(92)90082-e. [DOI] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Isolation and characterization of a bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1973 Oct;4(4):487–494. doi: 10.1128/aac.4.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Production and mode of action of lactocin 27: bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1975 Feb;7(2):139–145. doi: 10.1128/aac.7.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan E. E., Daly C., Fitzgerald G. F. Identification and characterization of helveticin V-1829, a bacteriocin produced by Lactobacillus helveticus 1829. J Appl Bacteriol. 1992 Oct;73(4):299–308. doi: 10.1111/j.1365-2672.1992.tb04981.x. [DOI] [PubMed] [Google Scholar]
- de Klerk H. C., Smit J. A. Properties of a Lactobacillus fermenti bacteriocin. J Gen Microbiol. 1967 Aug;48(2):309–316. doi: 10.1099/00221287-48-2-309. [DOI] [PubMed] [Google Scholar]




