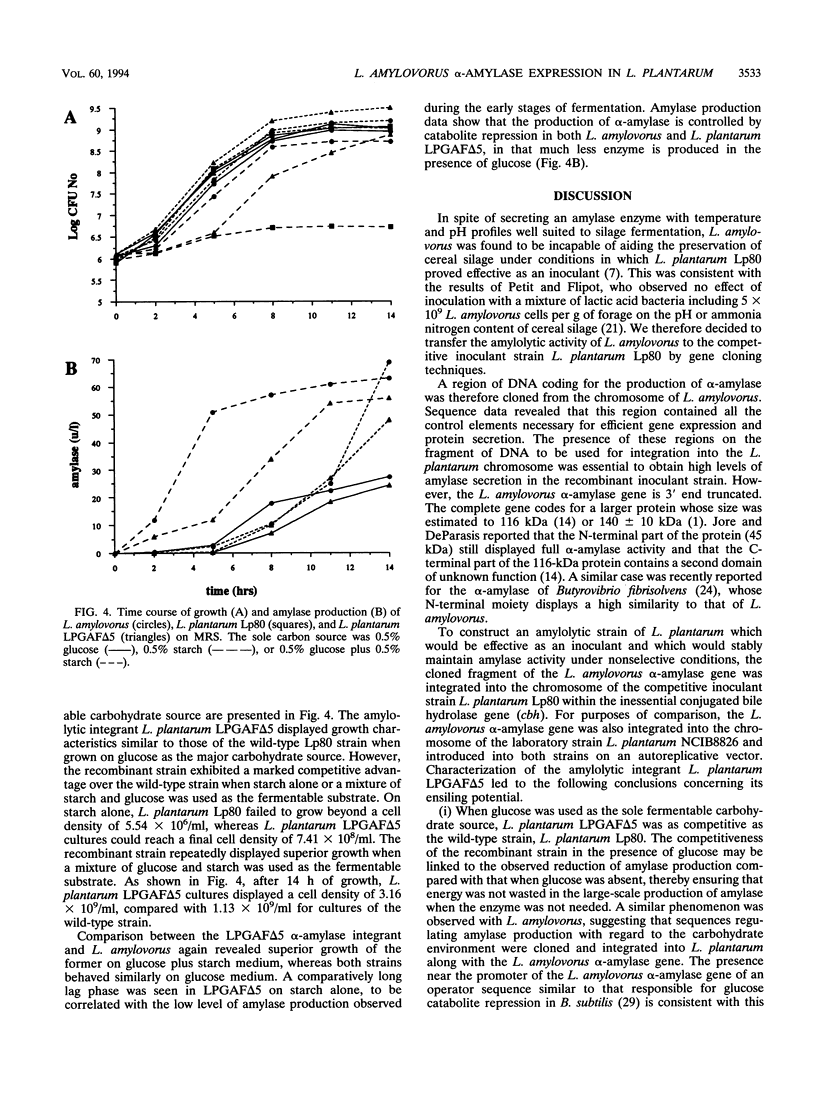
Abstract
An amylolytic Lactobacillus plantarum silage strain with the starch-degrading ability displayed by Lactobacillus amylovorus was developed. An active fragment of the gene coding for alpha-amylase production in L. amylovorus was cloned and integrated into the chromosome of the competitive inoculant strain L. plantarum Lp80 at the cbh locus. The alpha-amylase gene fragment was also introduced into L. plantarum Lp80 on an autoreplicative plasmid. Both constructions were also performed in the laboratory strain L. plantarum NCIB8826. All four recombinant strains secreted levels of amylase ranging from 23 to 69 U/liter, compared with 47 U/liter for L. amylovorus. Secretion levels were higher in L. plantarum NCIB8826 than in L. plantarum Lp80 derivatives and were higher in recombinant strains containing autoreplicative plasmids than in the corresponding integrants. The L. plantarum Lp80 derivative containing the L. amylovorus alpha-amylase gene fragment integrated into the host chromosome secreted alpha-amylase to a level comparable to that of L. amylovorus and was stable over 50 generations of growth under nonselective conditions. It grew to a higher cell density than either the parent strain or L. amylovorus in MRS medium containing a mixture of starch and glucose as the fermentable carbohydrate source. This recombinant alpha-amylolytic L. plantarum strain would therefore seem to have considerable potential as a silage inoculant for crops such as alfalfa, in which water-soluble carbohydrate levels are frequently low but starch is present as an alternative carbohydrate source.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J. D., Morrison D. A. Cloning of Streptococcus pneumoniae DNA fragments in Escherichia coli requires vectors protected by strong transcriptional terminators. Gene. 1987;55(2-3):179–187. doi: 10.1016/0378-1119(87)90278-2. [DOI] [PubMed] [Google Scholar]
- Christiaens H., Leer R. J., Pouwels P. H., Verstraete W. Cloning and expression of a conjugated bile acid hydrolase gene from Lactobacillus plantarum by using a direct plate assay. Appl Environ Microbiol. 1992 Dec;58(12):3792–3798. doi: 10.1128/aem.58.12.3792-3798.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocconcelli P. S., Gasson M. J., Morelli L., Bottazzi V. Single-stranded DNA plasmid, vector construction and cloning of Bacillus stearothermophilus alpha-amylase in Lactobacillus. Res Microbiol. 1991 Jul-Aug;142(6):643–652. doi: 10.1016/0923-2508(91)90077-n. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- Hols P., Ferain T., Garmyn D., Bernard N., Delcour J. Use of homologous expression-secretion signals and vector-free stable chromosomal integration in engineering of Lactobacillus plantarum for alpha-amylase and levanase expression. Appl Environ Microbiol. 1994 May;60(5):1401–1413. doi: 10.1128/aem.60.5.1401-1413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Warner P. J. Cloning and expression of alpha-amylase from Bacillus amyloliquefaciens in a stable plasmid vector in Lactobacillus plantarum. Lett Appl Microbiol. 1990 Oct;11(4):214–219. doi: 10.1111/j.1472-765x.1990.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Josson K., Scheirlinck T., Michiels F., Platteeuw C., Stanssens P., Joos H., Dhaese P., Zabeau M., Mahillon J. Characterization of a gram-positive broad-host-range plasmid isolated from Lactobacillus hilgardii. Plasmid. 1989 Jan;21(1):9–20. doi: 10.1016/0147-619x(89)90082-6. [DOI] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leer R. J., Christiaens H., Verstraete W., Peters L., Posno M., Pouwels P. H. Gene disruption in Lactobacillus plantarum strain 80 by site-specific recombination: isolation of a mutant strain deficient in conjugated bile salt hydrolase activity. Mol Gen Genet. 1993 May;239(1-2):269–272. doi: 10.1007/BF00281627. [DOI] [PubMed] [Google Scholar]
- Pouwels P. H., Leer R. J. Genetics of lactobacilli: plasmids and gene expression. Antonie Van Leeuwenhoek. 1993;64(2):85–107. doi: 10.1007/BF00873020. [DOI] [PubMed] [Google Scholar]
- Rumbak E., Rawlings D. E., Lindsey G. G., Woods D. R. Cloning, nucleotide sequence, and enzymatic characterization of an alpha-amylase from the ruminal bacterium Butyrivibrio fibrisolvens H17c. J Bacteriol. 1991 Jul;173(13):4203–4211. doi: 10.1128/jb.173.13.4203-4211.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheirlinck T., Mahillon J., Joos H., Dhaese P., Michiels F. Integration and expression of alpha-amylase and endoglucanase genes in the Lactobacillus plantarum chromosome. Appl Environ Microbiol. 1989 Sep;55(9):2130–2137. doi: 10.1128/aem.55.9.2130-2137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert M. J., Chambliss G. H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]




