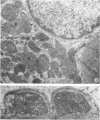
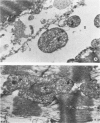
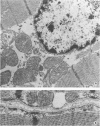
Abstract
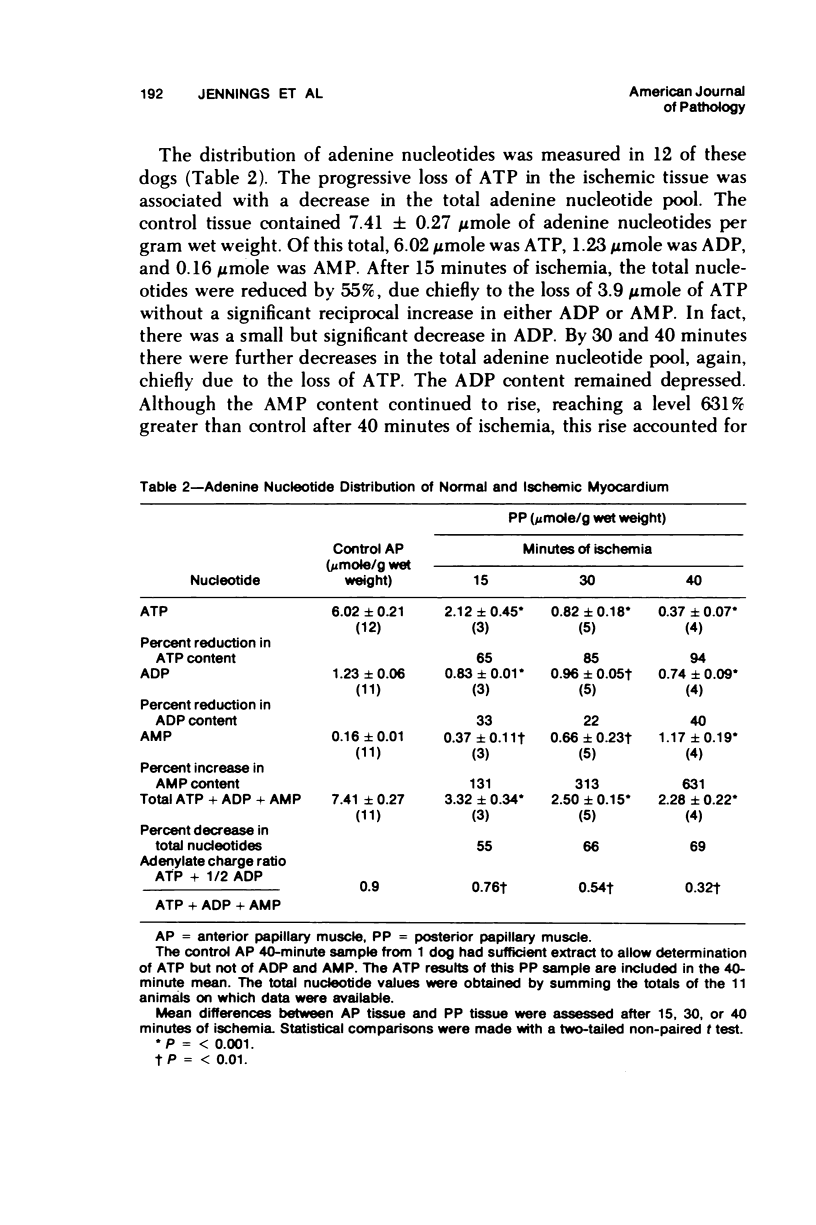
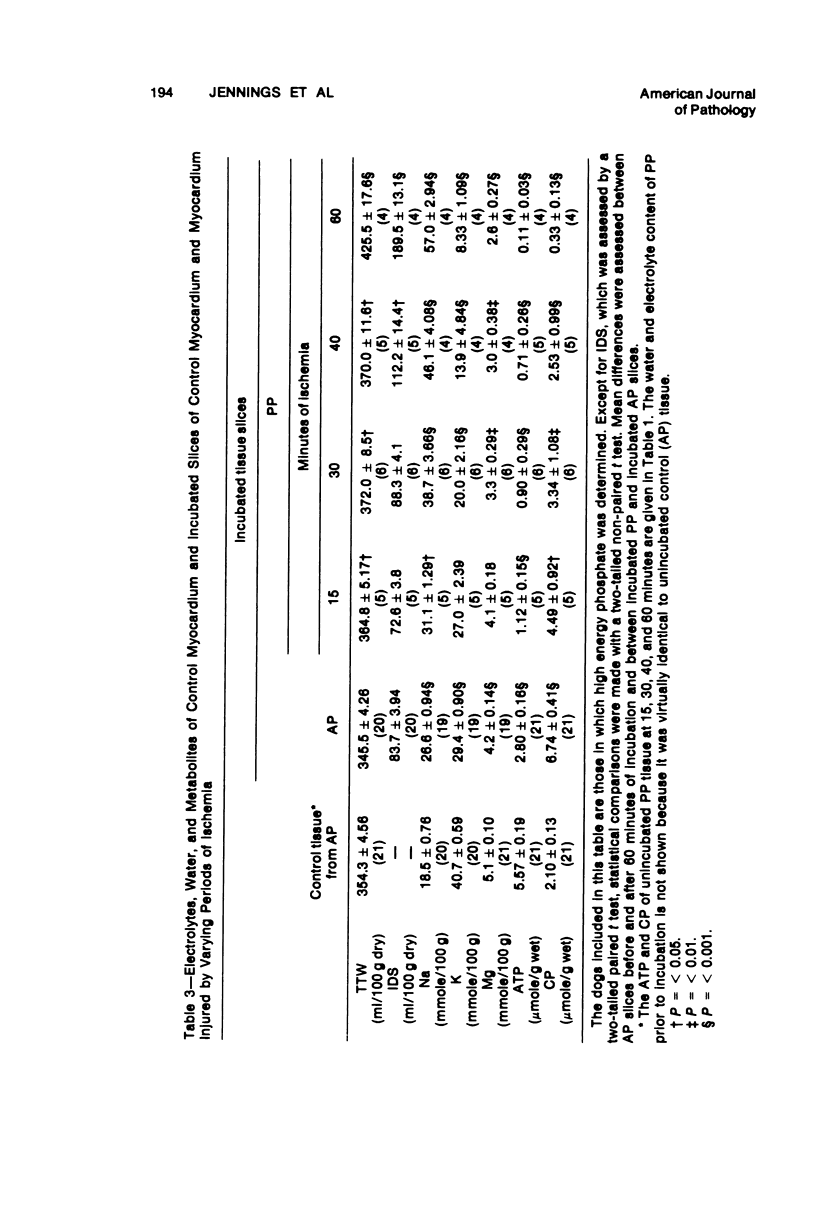
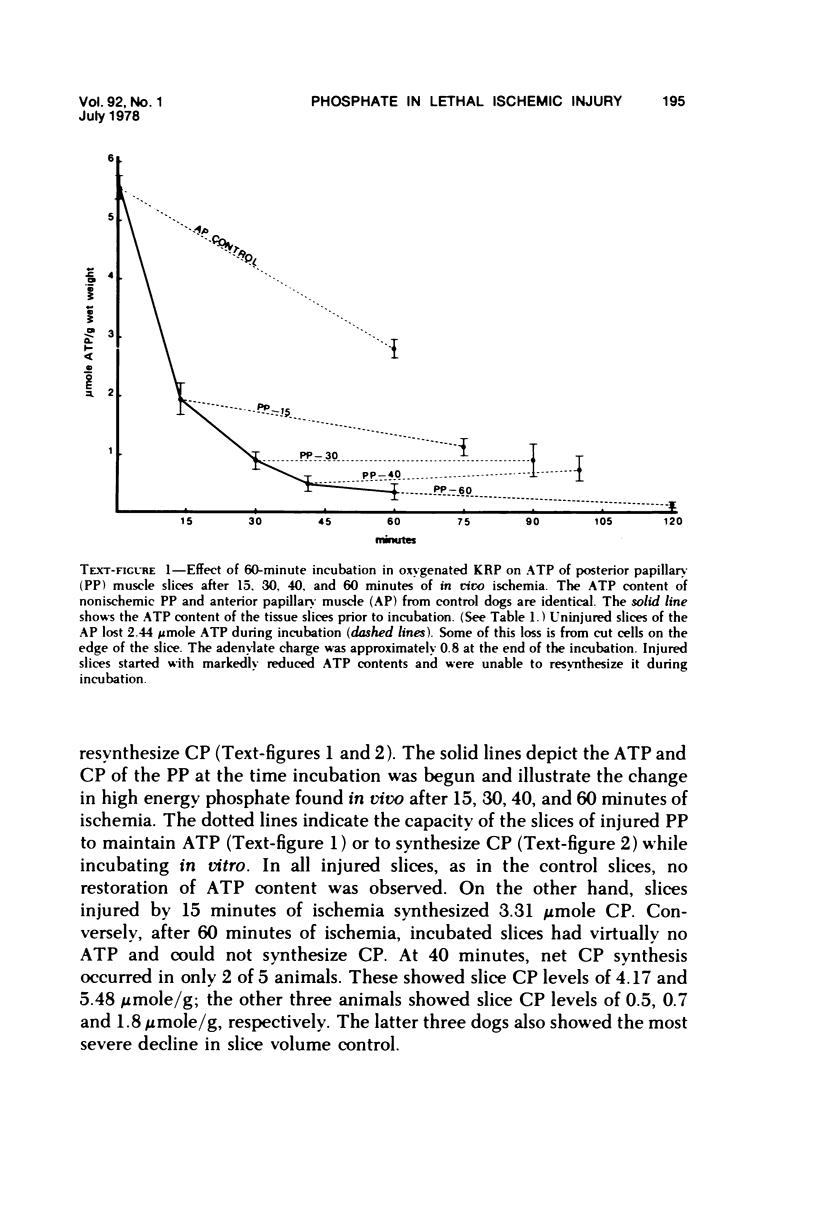
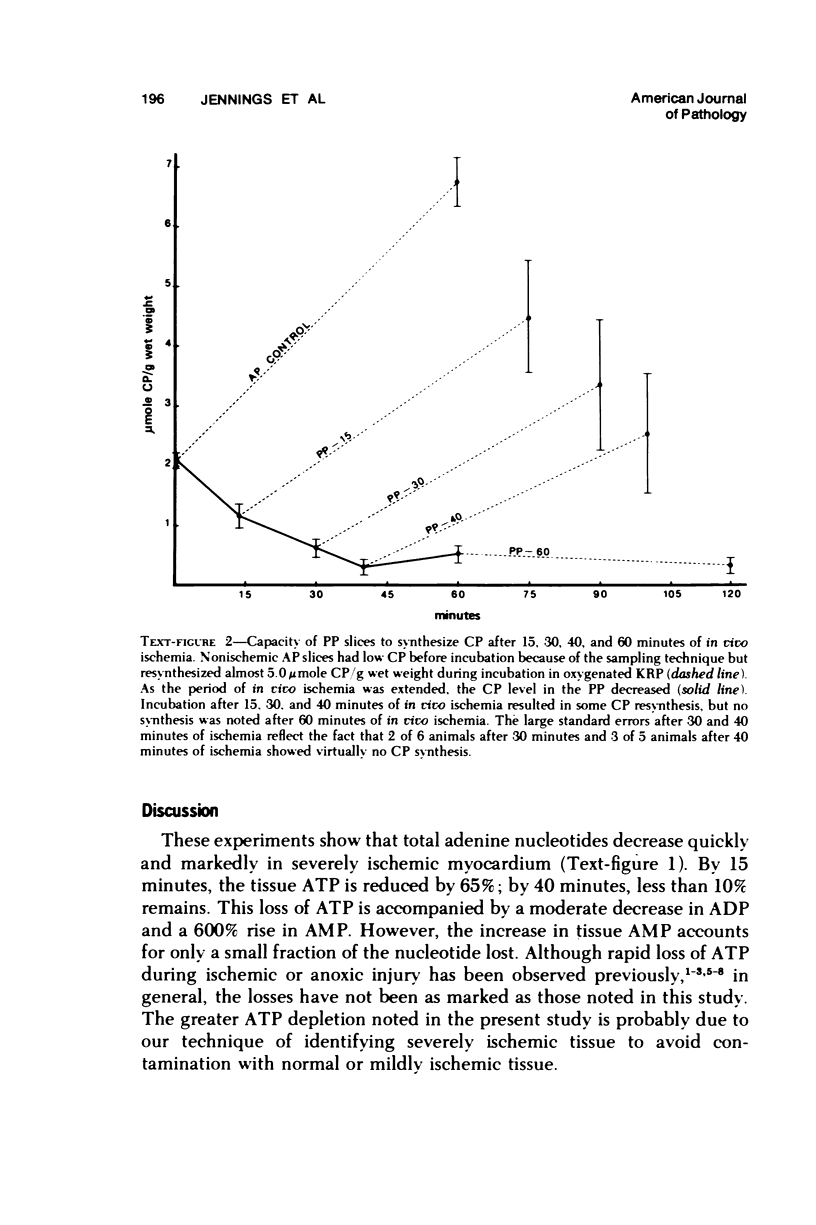
The relationship between progressive depletion of high energy phosphate and the onset of lethal cell injury in ischemic myocardium following coronary occlusion has been evaluated. Myocardial ischemia was induced by proximal occlusion of the circumflex coronary artery for 15, 30, 40, or 60 minutes. Cell injury in the severely ischemic posterior papillary muscle (PP) was evaluated by electron microscopy and by measuring the capacity of slices of the injured PP to maintain electrolytes, resynthesize high energy phosphate, and exclude inulin during in vitro incubation. ATP content in the ischemic myocardium decreased to 35%, 9%, 7%, and 5% of control values after 15, 30, 40, and 60 minutes of ischemia, respectively, and was associated with a corresponding depletion of total adenine nucleotides. The loss of 65% of the ATP after 15 minutes of ischemia (reversible injury) was associated with only minimal ultrastructural changes and no significant defects of electrolytes in incubated slices. However, the depletion of over 90% of the ATP after 40 minutes of ischemia (irreversible injury) was associated with significant fine structural changes and markedly altered cell volume regulation. The results suggest a close relationship between the marked depletion of high energy phsophates and the development of lethal injury in acutely ischemic myocardium.
Full text
PDF



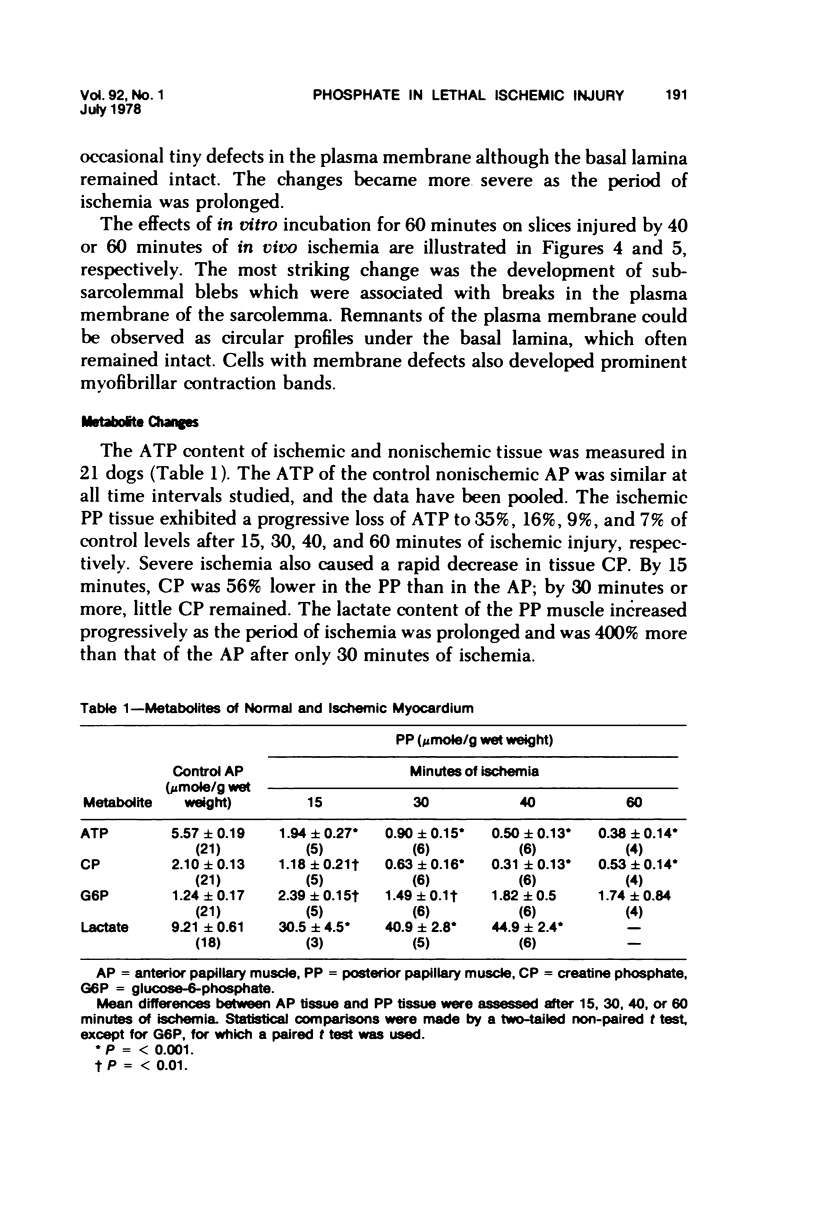











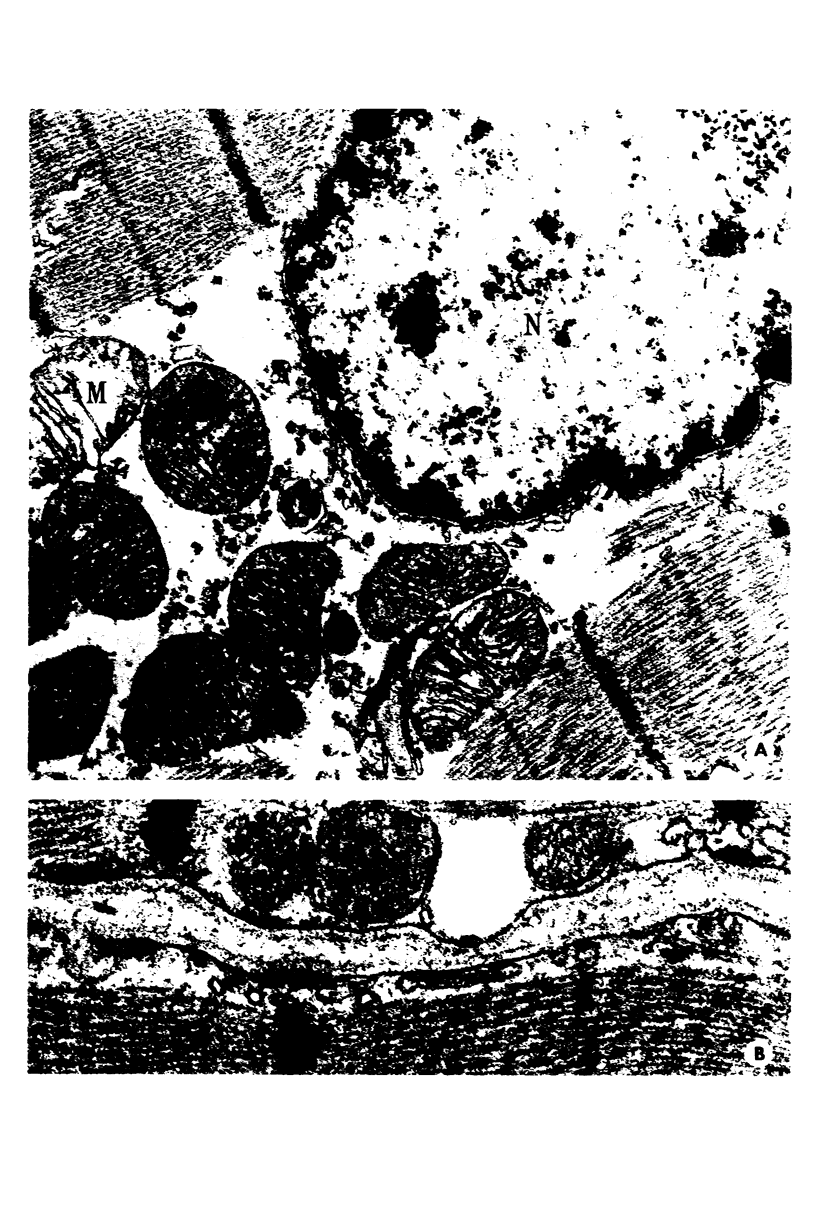

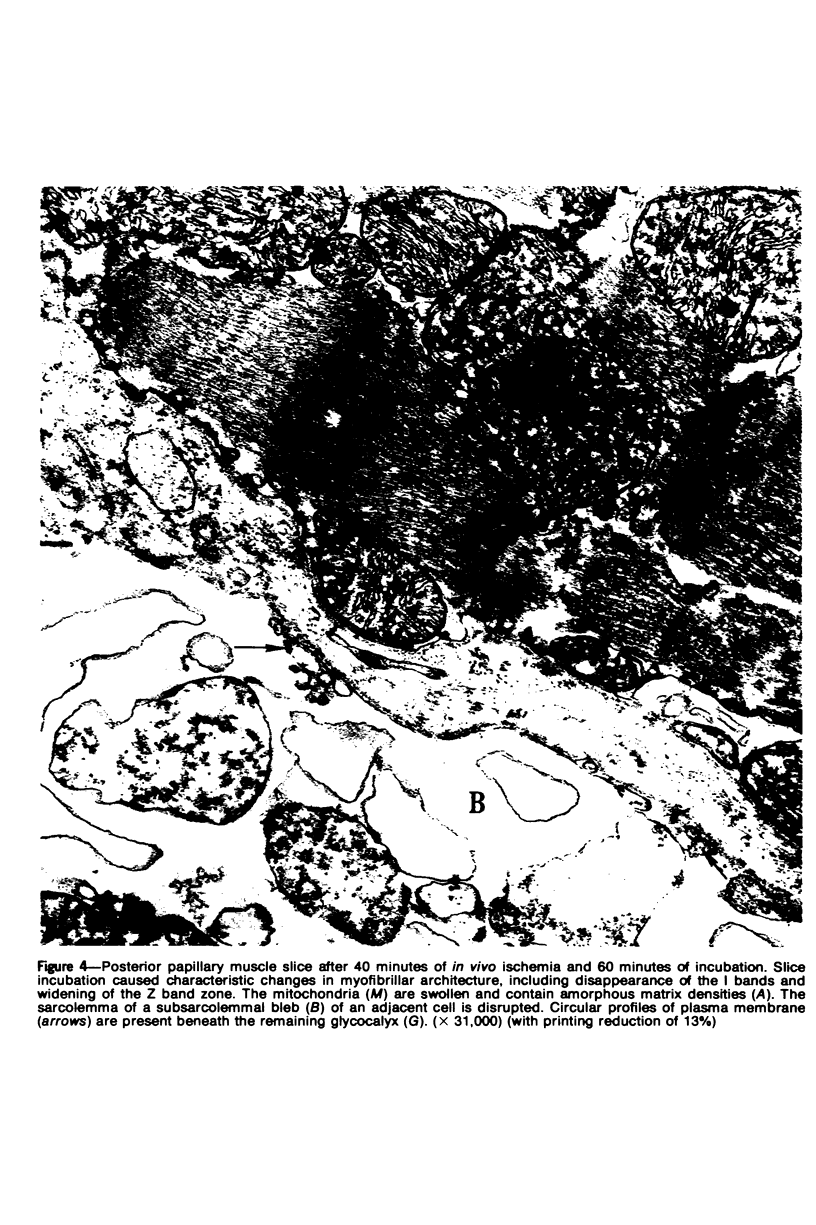
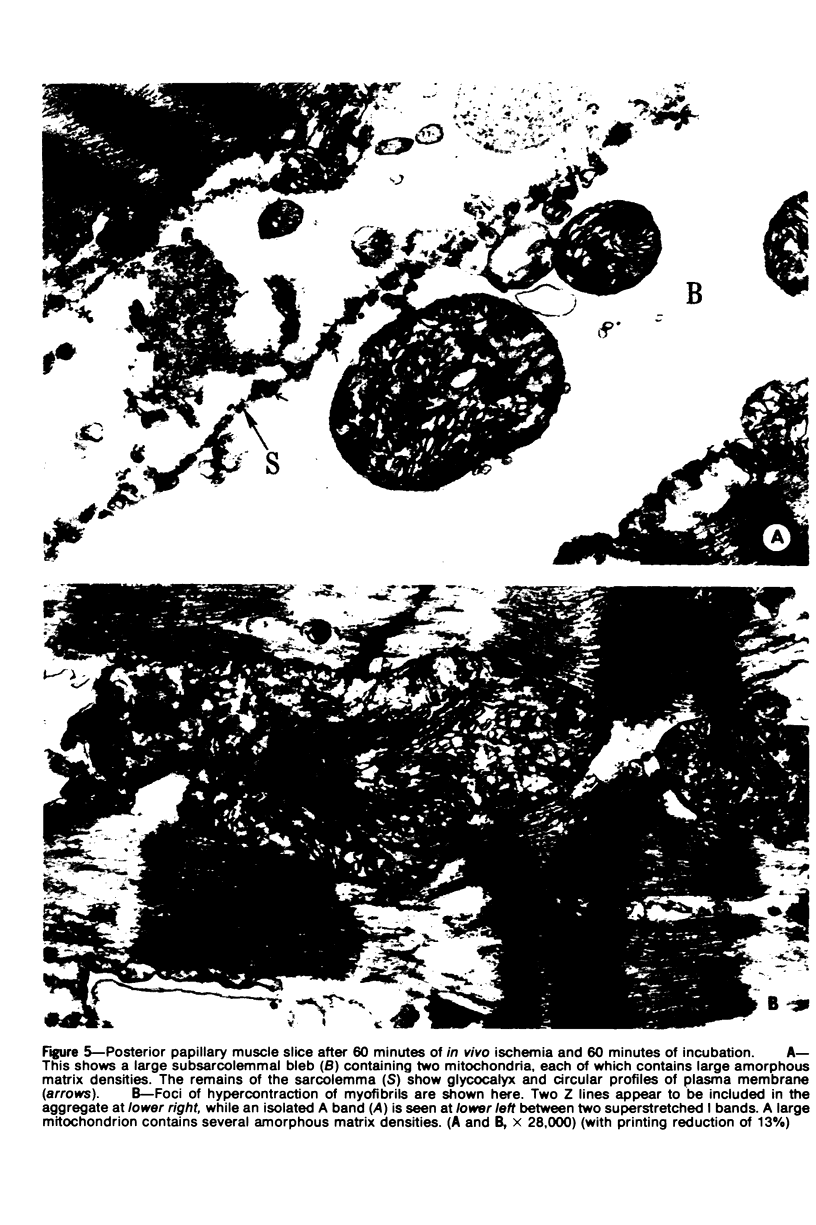
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison T. B., Ramey C. A., Holsinger J. W., Jr Transmural gradients of left ventricular tissue metabolites after circumflex artery ligation in dogs. J Mol Cell Cardiol. 1977 Oct;9(10):837–852. doi: 10.1016/s0022-2828(77)80060-6. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- BUSCH E. W., HABEL G., VON WICHERT RESTITUTION VON ADENOSINPHOSPHATEN IM LEBERGEWEBE WAEHREND DER WIEDERDURCHBLUTUNG NACH ISCHAEMISCHEN BELASTUNGEN BIS ZU 5 STD DAUER. Biochem Z. 1964 Dec 7;341:85–96. [PubMed] [Google Scholar]
- Berne R. M., Rubio R. Adenine nucleotide metabolism in the heart. Circ Res. 1974 Sep;35 (Suppl 3):109–120. [PubMed] [Google Scholar]
- Dobson J. G., Jr, Mayer S. E. Mechanisms of activation of cardiac glycogen phosphorylase in ischemia and anoxia. Circ Res. 1973 Oct;33(4):412–420. doi: 10.1161/01.res.33.4.412. [DOI] [PubMed] [Google Scholar]
- Dunn R. B., Griggs D. M., Jr Transmural gradients in ventricular tissue metabolites produced by stopping coronary blood flow in the dog. Circ Res. 1975 Oct;37(4):438–445. doi: 10.1161/01.res.37.4.438. [DOI] [PubMed] [Google Scholar]
- Farber E. ATP and cell integrity. Fed Proc. 1973 Apr;32(4):1534–1539. [PubMed] [Google Scholar]
- Farber J. L., El-Mofty S. K. The biochemical pathology of liver cell necrosis. Am J Pathol. 1975 Oct;81(1):237–250. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Jennings R. B., Hill M. L., Grochowski E. Experimental myocardial ischemic injury. II. Effect of in vivo ischemia on dog heart slice function in vitro. J Mol Cell Cardiol. 1976 Mar;8(3):189–204. doi: 10.1016/0022-2828(76)90036-5. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Loyter A., Reichler Y., Ohad I. Correlation between changes in the membrane organization and susceptibility to phospholipase C attack induced by ATP depletion of rat erythrocytes. Biochim Biophys Acta. 1976 Feb 6;419(3):479–492. doi: 10.1016/0005-2736(76)90260-1. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Ohad I., Loyter A. Phosphorylation and dephosphorylation of membrane proteins as a possible mechanism for structural rearrangement of membrane components. Biochim Biophys Acta. 1976 Jun 4;436(1):1–14. doi: 10.1016/0005-2736(76)90214-5. [DOI] [PubMed] [Google Scholar]
- Ginn F. L., Shelburne J. D., Trump B. F. Disorders of cell volume regulation. I. Effects of inhibition of plasma membrane adenosine triphosphatase with ouabain. Am J Pathol. 1968 Dec;53(6):1041–1071. [PMC free article] [PubMed] [Google Scholar]
- Grochowski E. C., Ganote C. E., Hill M. L., Jennings R. B. Experimental myocardial ischemic injury. I. A comparison of Stadie-Riggs and free-hand slicing techniques on tissue ultrastructure, water and electrolytes during in vitro incubation. J Mol Cell Cardiol. 1976 Mar;8(3):173–187. doi: 10.1016/0022-2828(76)90035-3. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S., Mathes P., Ravens K. G. Functional compartmentation of ATP and creatine phosphate in heart muscle. J Mol Cell Cardiol. 1970 Sep;1(3):325–339. doi: 10.1016/0022-2828(70)90009-x. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Stewart D. A., Chain E. B. Diabetes and the survival and recovery of the anoxic myocardium. J Mol Cell Cardiol. 1975 Jun;7(6):397–415. doi: 10.1016/0022-2828(75)90046-2. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., DeLuca M., Sybers H. D., Wildenthal K. Fetal mouse hearts: a model for studying ischemia. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2809–2813. doi: 10.1073/pnas.72.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNINGS R. B., CROUT J. R., SMETTERS G. W. Studies on distribution and localization to potassium in early myocardial ischemic injury. AMA Arch Pathol. 1957 Jun;63(6):586–592. [PubMed] [Google Scholar]
- JENNINGS R. B., SOMMERS H. M., SMYTH G. A., FLACK H. A., LINN H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960 Jul;70:68–78. [PubMed] [Google Scholar]
- JENNINGS R. B., WARTMAN W. B. Production of an area of homogeneous myocardial infarction in the dog. AMA Arch Pathol. 1957 Jun;63(6):580–585. [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E., Reimer K. A. Ischemic tissue injury. Am J Pathol. 1975 Oct;81(1):179–198. [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jennings R. B., Moore C. B., Shen A. C., Herdson P. B. Electrolytes of damaged myocardial mitochondria. Proc Soc Exp Biol Med. 1970 Nov;135(2):515–522. doi: 10.3181/00379727-135-35086. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- Jones C. E., Thomas J. X., Parker J. C., Parker R. E. Acute changes in high energy phosphates, nucleotide derivatives, and contractile force in ischaemic and nonischaemic canine myocardium following coronary occlusion. Cardiovasc Res. 1976 May;10(3):275–282. doi: 10.1093/cvr/10.3.275. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Hossmann K. A., Pegg A. E., Kobayashi K., Zimmermann V. Resuscitation of the monkey brain after one hour complete ischemia. III. Indications of metabolic recovery. Brain Res. 1975 Sep 12;95(1):61–73. doi: 10.1016/0006-8993(75)90207-3. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Kobayashi K., Hossmann K. A. Purine nucleotide metabolism in the cat brain after one hour of complete ischemia. J Neurochem. 1974 Aug;23(2):417–425. doi: 10.1111/j.1471-4159.1974.tb04374.x. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Reimer K. A., Jennings R. B. Distribution of coronary arterial flow in acute myocardial ischemia. Arch Pathol. 1975 Feb;99(2):86–94. [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Kloner R. A., Reimer K. A., Jennings R. B. Distribution of coronary collateral flow in acute myocardial ischaemic injury: effect of propranolol. Cardiovasc Res. 1976 Jan;10(1):81–90. doi: 10.1093/cvr/10.1.81. [DOI] [PubMed] [Google Scholar]
- Kübler W., Spieckermann P. G. Regulation of glycolysis in the ischemic and the anoxic myocardium. J Mol Cell Cardiol. 1970 Dec;1(4):351–377. doi: 10.1016/0022-2828(70)90034-9. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho K. U., Trump B. F. Relationship of ionic, water, and cell volume changes in cellular injury of Ehrlich ascites tumor cells. Lab Invest. 1974 Sep;31(3):207–215. [PubMed] [Google Scholar]
- Laiho K. U., Trump B. F. Studies on the pathogenesis of cell injury: effects of inhibitors of metabolism and membrane function on the mitochondria of Ehrlich ascites tumor cells. Lab Invest. 1975 Feb;32(2):163–182. [PubMed] [Google Scholar]
- Nayler W. G., Grau A., Slade A. A protective effect of verapamil on hypoxic heart muscle. Cardiovasc Res. 1976 Nov;10(6):650–662. doi: 10.1093/cvr/10.6.650. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Whitmer J. T., Morgan H. E. Effects of ischemia on function and metabolism of the isolated working rat heart. Am J Physiol. 1973 Sep;225(3):651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- Pool P. E., Covell J. W., Chidsey C. A., Braunwald E. Myocardial high energy phosphate stores in acutely induced hypoxic heart failure. Circ Res. 1966 Aug;19(2):221–229. doi: 10.1161/01.res.19.2.221. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Lowe J. E., Jennings R. B. Effect of the calcium antagonist verapamil on necrosis following temporary coronary artery occlusion in dogs. Circulation. 1977 Apr;55(4):581–587. doi: 10.1161/01.cir.55.4.581. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Rasmussen M. M., Jennings R. B. Reduction by propranolol of myocardial necrosis following temporary coronary artery occlusion in dogs. Circ Res. 1973 Sep;33(3):353–363. doi: 10.1161/01.res.33.3.353. [DOI] [PubMed] [Google Scholar]
- Ridge J. W. Hypoxia and the energy charge of the cerebral adenylate pool. Biochem J. 1972 Apr;127(2):351–355. doi: 10.1042/bj1270351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovetto M. J., Whitmer J. T., Neely J. R. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ Res. 1973 Jun;32(6):699–711. doi: 10.1161/01.res.32.6.699. [DOI] [PubMed] [Google Scholar]
- SAYEN J. J., SHELDON W. F., PEIRCE G., KUO P. T. Polarographic oxygen, the epicardial electrocardiogram and muscle contraction in experimental acute regional ischemia of the left ventricle. Circ Res. 1958 Nov;6(6):779–798. doi: 10.1161/01.res.6.6.779. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Stezoski S. W. Protective role of increased myocardial glycogen stores in cardiac anoxia in the rat. Circ Res. 1970 Nov;27(5):835–849. doi: 10.1161/01.res.27.5.835. [DOI] [PubMed] [Google Scholar]
- Scheuer J. The effect of hypoxia on glycolytic ATP production. J Mol Cell Cardiol. 1972 Dec;4(6):689–692. doi: 10.1016/0022-2828(72)90122-8. [DOI] [PubMed] [Google Scholar]
- Sommers H. M., Jennings R. B. Ventricular fibrillation and myocardial necrosis after transient ischemia. Effect of treatment with oxygen, procainamide, reserpine, and propranolol. Arch Intern Med. 1972 May;129(5):780–789. [PubMed] [Google Scholar]
- Thomas R. A., Rubio R., Berne R. M. Comparison of the adenine nucleotide metabolism of dog atrial and ventricular myocardium. J Mol Cell Cardiol. 1975 Feb;7(2):115–123. doi: 10.1016/0022-2828(75)90013-9. [DOI] [PubMed] [Google Scholar]
- Vogt M. T., Farber E. On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol. 1968 Jul;53(1):1–26. [PMC free article] [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]
- Williamson J. R., Schaffer S. W., Ford C., Safer B. Contribution of tissue acidosis to ischemic injury in the perfused rat heart. Circulation. 1976 Mar;53(3 Suppl):I3–14. [PubMed] [Google Scholar]
- Wollenberger A., Krause E. G. Metabolic control characteristics of the acutely ischemic myocardium. Am J Cardiol. 1968 Sep;22(3):349–359. doi: 10.1016/0002-9149(68)90119-7. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Hutchings A. E., Brachfeld N. Lipid metabolism in myocardial cell-free homogenates. J Mol Cell Cardiol. 1972 Apr;4(2):97–111. doi: 10.1016/0022-2828(72)90068-5. [DOI] [PubMed] [Google Scholar]