Abstract
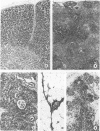
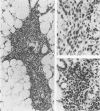
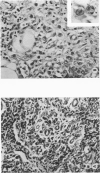
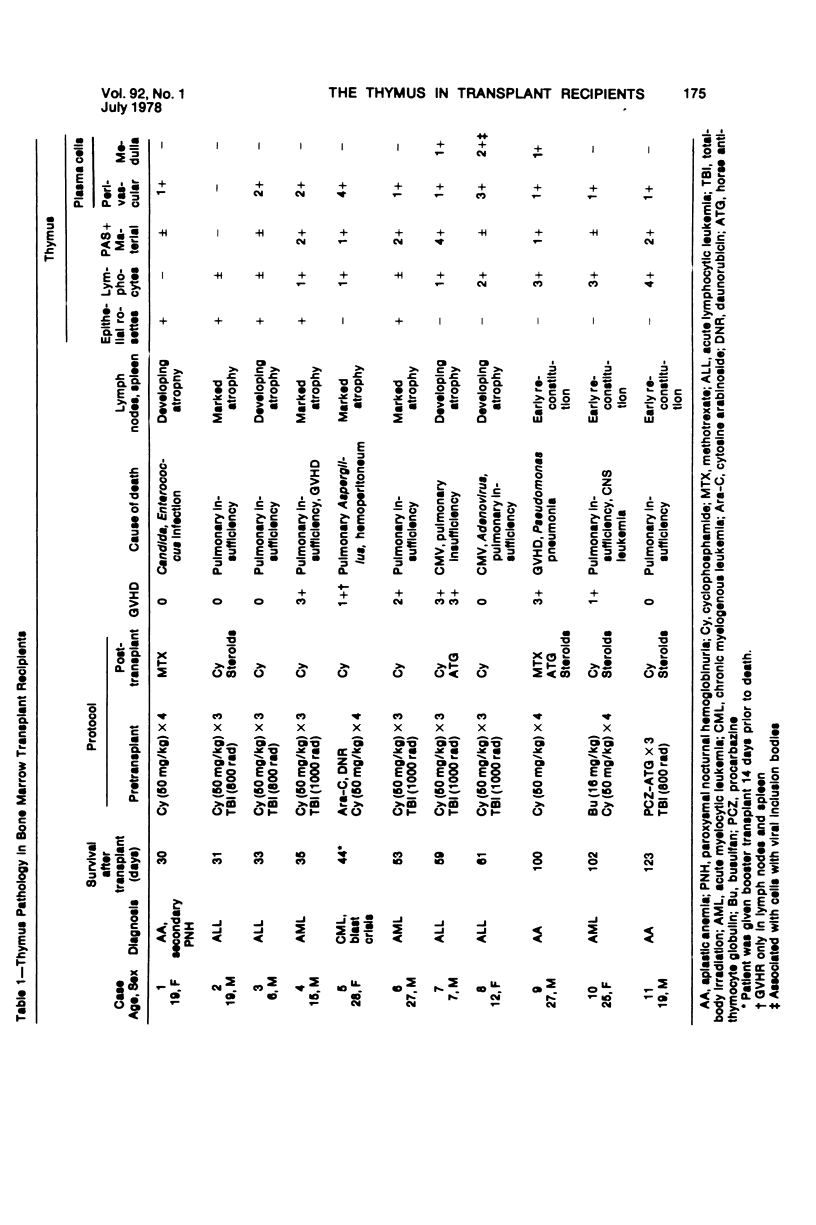
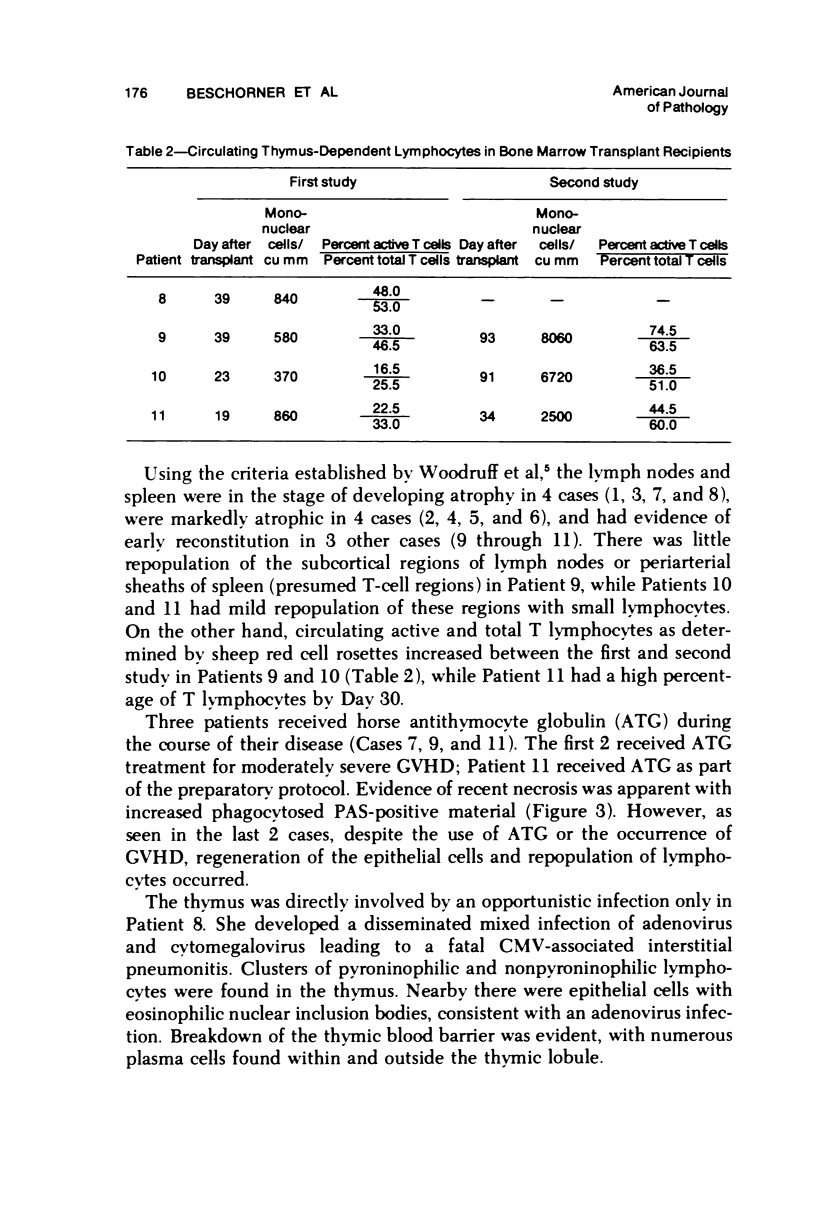
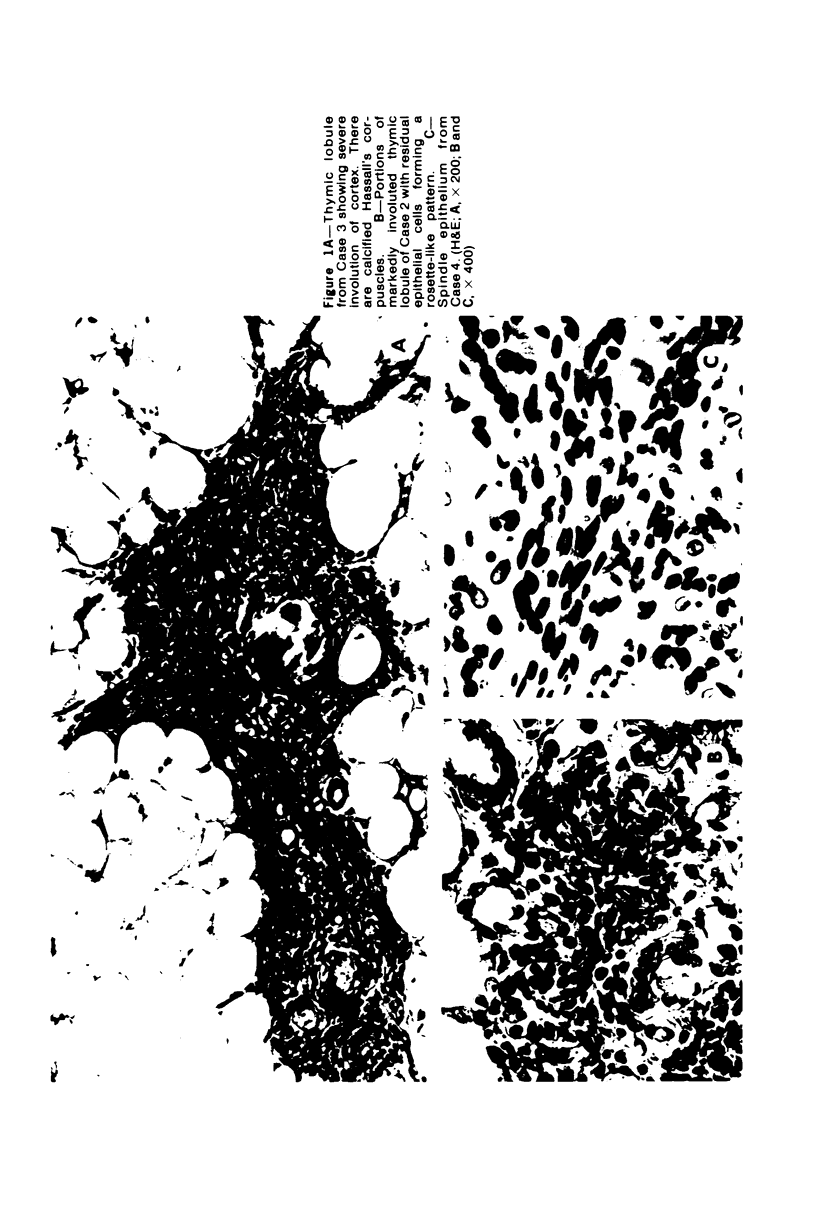
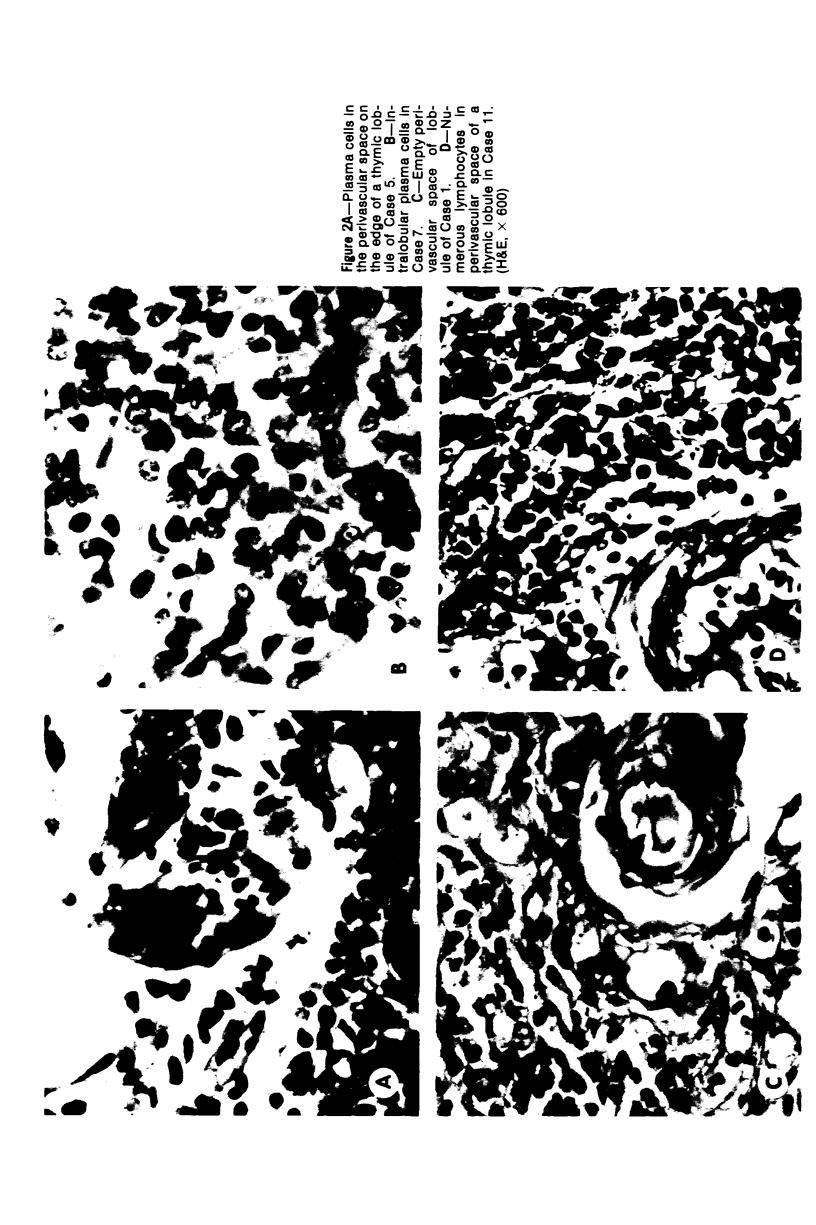
The thymus glands from 11 patients with aplastic anemia or acute leukemia who received allogeneic bone marrow transplants were studied at autopsy. All showed marked cortical involution. In the short-term survivors the medulla and perivascular spaces were lymphocyte-depleted and the epithelial cells formed pseudorosettes. In those surviving over 2 months, increasing numbers of small lymphocytes were present, presumably reconstituted with donor lymphocytes. Phagocytosis of cellular debris was frequent, especially in patients with graft-versus-host reaction (GVHR) or treated with anithymocyte globulin (ATG). Plasma cells were numerous in perilobular tissue and were occasionally found within the medulla. The findings are compatible with the concept that the thymus plays an important role in the immune deficiency experienced after allogeneic bone marrow transplantation and in the subsequent lymphoid reconstitution.
Full text
PDF







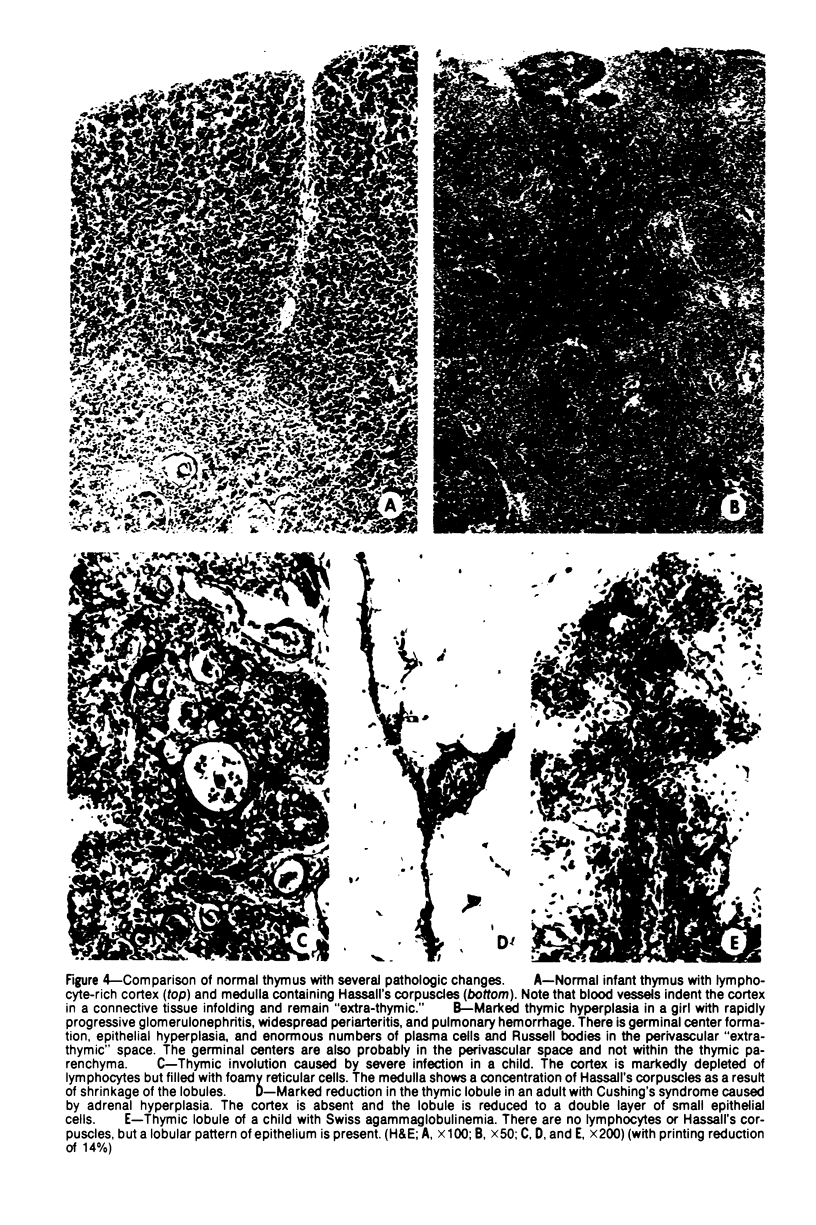
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Kawamoto Y., Furuta I., Kondo T. Early morphological changes in cortical medullary thymocytes of the rat after whole-body irradiation. I. Electron-microscope observations. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Jun;21(6):545–558. doi: 10.1080/09553007214550641. [DOI] [PubMed] [Google Scholar]
- BURNET F. M., MACKAY I. R. Lymphoepithelial structures and autoimmune disease. Lancet. 1962 Nov 17;2(7264):1030–1033. doi: 10.1016/s0140-6736(62)92710-1. [DOI] [PubMed] [Google Scholar]
- Elfenbein G. J., Anderson P. N., Humphrey R. L., Mullins G. M., Sensenbrenner L. L., Wands J. R., Santos G. W. Immune system reconstitution following allogeneic bone marrow transplantation in man: a multiparameter analysis. Transplant Proc. 1976 Dec;8(4):641–646. [PubMed] [Google Scholar]
- HUTCHINS G. M., HARVEY A. M. THE THYMUS IN SYSTEMIC LUPUS ERYTHEMATOSUS. Bull Johns Hopkins Hosp. 1964 Nov;115:355–378. [PubMed] [Google Scholar]
- Halterman R. H., Graw R. G., Jr, Fuccillo D. A., Leventhal B. G. Immunocompetence following allogeneic bone marrow transplantation in man. Transplantation. 1972 Dec;14(6):689–697. doi: 10.1097/00007890-197212000-00004. [DOI] [PubMed] [Google Scholar]
- Kateley J. R., Gengozian N. Rat-mouse radiation chimeras: characterization of an antibody-mediated graft-vs-host reaction. J Immunol. 1975 Jan;114(1 Pt 1):125–132. [PubMed] [Google Scholar]
- Kersey J. H., Meuwissen H. J., Good R. A. Graft versus host reactions following transplantation of allogeneic hematopoietic cells. Hum Pathol. 1971 Sep;2(3):389–402. doi: 10.1016/s0046-8177(71)80006-0. [DOI] [PubMed] [Google Scholar]
- Merritt C. B., Mann D. L., Rogentine G. N., Jr Cytotoxic antibody for epithelial cells in human graft versus host disease. Nature. 1971 Aug 27;232(5313):638–639. doi: 10.1038/232638a0. [DOI] [PubMed] [Google Scholar]
- Raviola E., Karnovsky M. J. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972 Sep 1;136(3):466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainte-Marie G., Peng F. S. Emigration of thymocytes from the thymus: a review and study of the problem. Rev Can Biol. 1971 Mar;30(1):51–78. [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977 Jul;88(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Bradley J., Daly D., Ziff M. Immunoglobulin synthesis in vitro by human thymus: comparison of myasthenia gravis and normal thymus. Clin Exp Immunol. 1969 Apr;4(4):387–399. [PMC free article] [PubMed] [Google Scholar]
- Storb R., Ochs H. D., Weiden P. L., Thomas E. D. Immunologic reactivity in marrow graft recipients. Transplant Proc. 1976 Dec;8(4):637–639. [PubMed] [Google Scholar]
- Swasdikul D., Block M. Effect of radiation upon the "embryonic" thymus. Radiat Res. 1972 Apr;50(1):73–84. [PubMed] [Google Scholar]
- Thomas E. D., Storb R., Clift R. A., Fefer A., Johnson L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (second of two parts). N Engl J Med. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- Thomas E., Storb R., Clift R. A., Fefer A., Johnson F. L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975 Apr 17;292(16):832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- Woodruff J. M., Hansen J. A., Good R. A., Santos G. W., Slavin R. E. The pathology of the graft-versus-host reaction (GVHR) in adults receiving bone marrow transplants. Transplant Proc. 1976 Dec;8(4):675–684. [PubMed] [Google Scholar]