Abstract
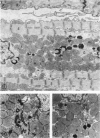
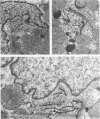
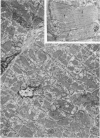
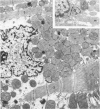
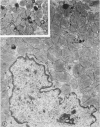
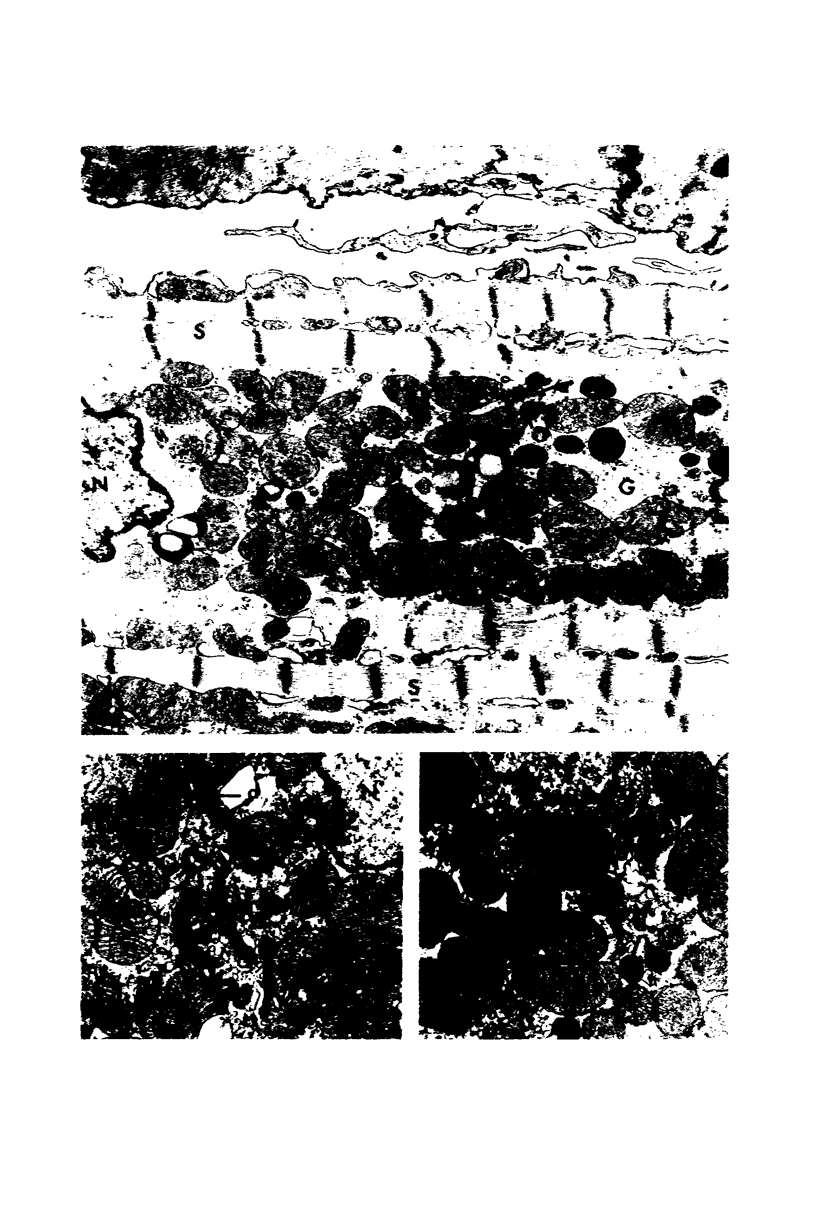
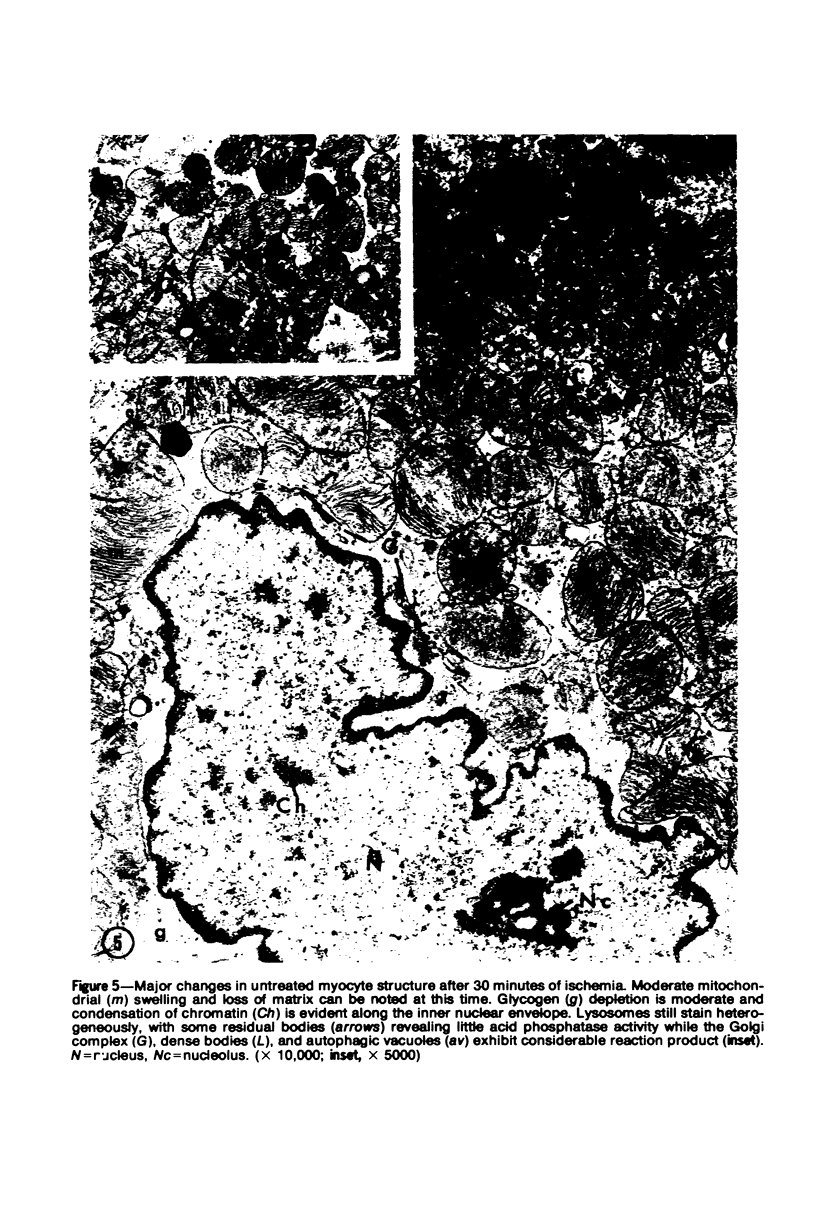
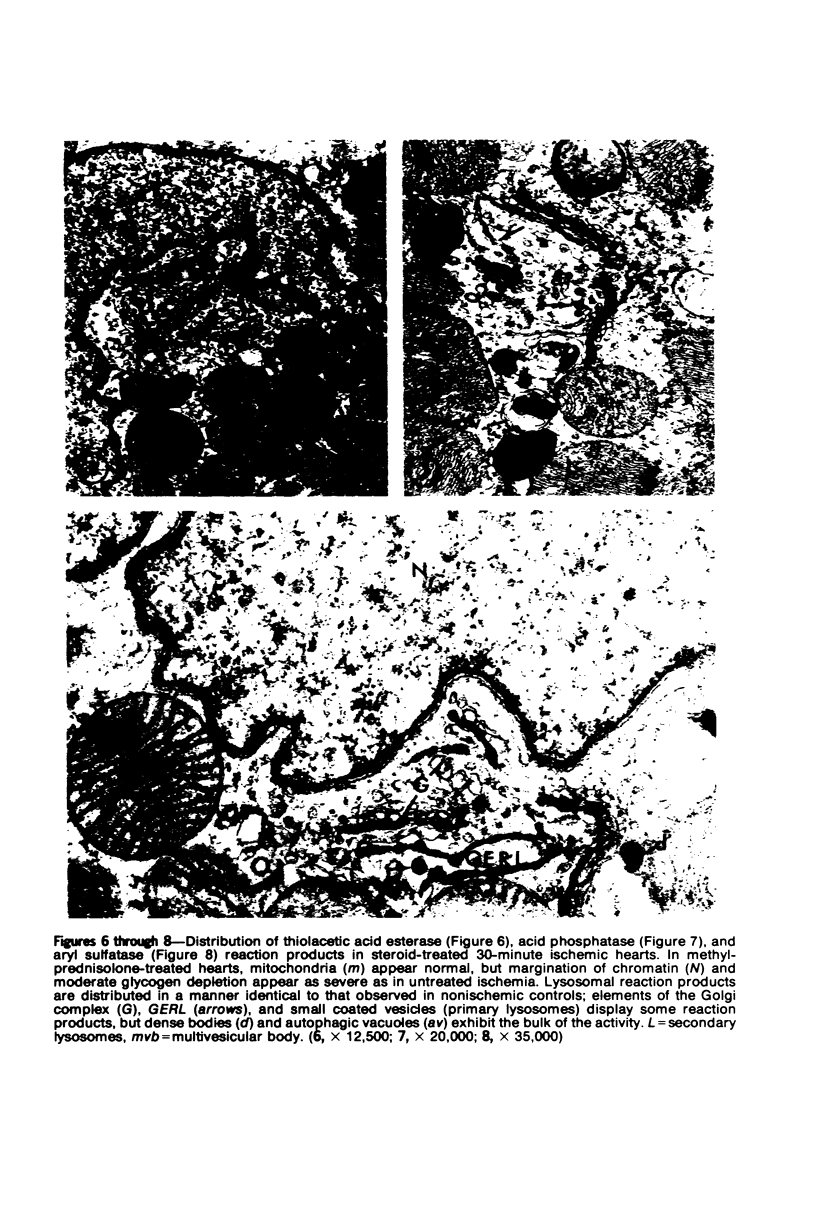
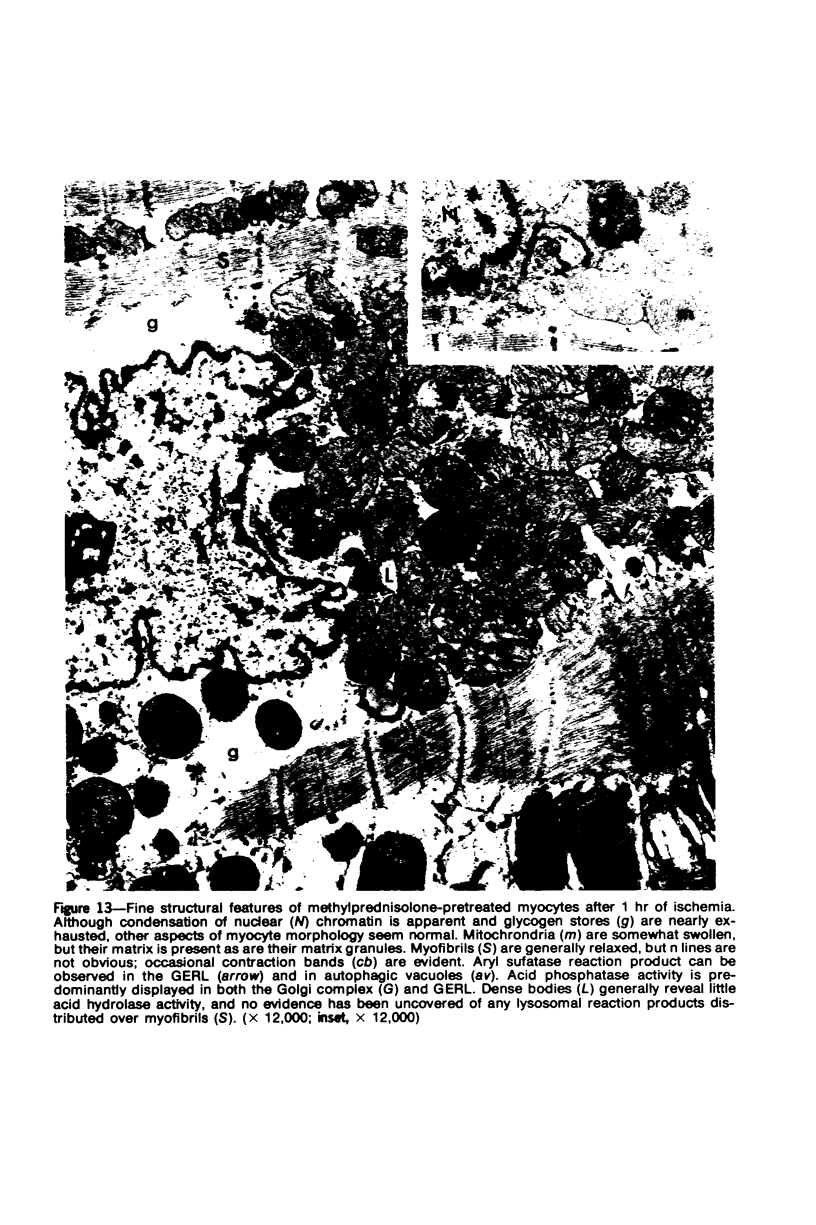
Occlusion of the circumflex branch of the coronary artery of rabbit hearts for 45 minutes elicits structural and cytochemical changes in myocytes similar to those observed in ischemic dog myocardium, which are indicative of irreversible cell injury. When methylprednisolone is administered prior to occluding the artery, myocytes are transiently protected and many of the electron microscopic signs of irreversible damage are delayed for 15 minutes or more. During this period, the steroid preferentially protects mitochondria, lysosomes, and sarcolemma from the ischemic changes that normally develop. However, some other events, including depletion of glycogen and margination of nuclear chromatin, are only minimally influenced by the therapy, if at all. In all hearts, treated and untreated, the development of severe cell damage, whenever it occurs, is closely associated with cell swelling, mitochondrial dilation with concomitant appearance of amorphous osmiophilic densities, and abnormalities in and, ultimately disappearance of lysosomes, suggesting that damage to cell membranes is a central event in the progression of reversible injury to irreversible infarction and that protection of membrane integrity should be a reasonable aim in efforts to ameliorate or delay ischemic injury.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Ellis A., Glick G. Effects of methylprednisolone on cardiac lymph in acute myocardial ischemia in dogs. Am J Physiol. 1977 Jun;232(6):H602–H607. doi: 10.1152/ajpheart.1977.232.6.H602. [DOI] [PubMed] [Google Scholar]
- Bell M., Barrnett R. J. The use of thiol-substituted carboxylic acids as histochemical substrates. J Histochem Cytochem. 1965 Nov-Dec;13(8):611–628. doi: 10.1177/13.8.611. [DOI] [PubMed] [Google Scholar]
- Brachfeld N. Metabolic evaluation of agents designed to protect the ischemic myocardium and to reduce infarct size. Am J Cardiol. 1976 Mar 31;37(4):528–532. doi: 10.1016/0002-9149(76)90392-1. [DOI] [PubMed] [Google Scholar]
- Brunk U. T., Ericsson J. L. Cytochemical evidence for the leakage of acid phosphatase through ultrastructurally intact lysosomal membranes. Histochem J. 1972 Nov;4(6):479–491. doi: 10.1007/BF01011128. [DOI] [PubMed] [Google Scholar]
- Busuttil R. W., George W. J., Hewitt R. L. Protective effect of methylprednisolone on the heart during ischemic arrest. J Thorac Cardiovasc Surg. 1975 Dec;70(6):955–965. [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Griffin E. E., Dingle J. T., Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977 May;59(5):911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola M., Rovetto M., Soriano R., Cho S. Y., Wiener L. Glucocorticoid protection of the myocardial cell membrane and the reduction of edema in experimental acute myocardial ischemia. J Thorac Cardiovasc Surg. 1976 Oct;72(4):631–643. [PubMed] [Google Scholar]
- Goldfischer S. The cytochemical demonstration of lysosomal aryl sulfatase activity by light and electron microscopy. J Histochem Cytochem. 1965 Jul-Aug;13(6):520–523. doi: 10.1177/13.6.520. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Letter: Pacing the WPW patient. Circulation. 1976 Jan;53(1):204–205. doi: 10.1161/01.cir.53.1.204. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M. Enzyme release during myocardial anoxia: a study of metabolic protection. J Mol Cell Cardiol. 1975 Jul;7(7):463–482. doi: 10.1016/0022-2828(75)90164-9. [DOI] [PubMed] [Google Scholar]
- Hopsu-Havu V. K., Arstila A. U., Helminen H. J., Kalimo H. O. Improvements in the method for the electron microscopic localization of arylsulphatase activity. Histochemie. 1967;8(1):54–64. doi: 10.1007/BF00279874. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., DeLuca M., Sybers H. D., Wildenthal K. Fetal mouse hearts: a model for studying ischemia. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2809–2813. doi: 10.1073/pnas.72.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- Kidwai A. M., Radcliffe M. A., Duchon G., Daniel E. E. Isolation of plasma membrane from cardiac muscle. Biochem Biophys Res Commun. 1971 Nov;45(4):901–910. doi: 10.1016/0006-291x(71)90423-2. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Lew H., Maroko P. R., Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 1978 Jan;57(1):56–63. doi: 10.1161/01.cir.57.1.56. [DOI] [PubMed] [Google Scholar]
- Libby P., Maroko P. R., Bloor C. M., Sobel B. E., Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest. 1973 Mar;52(3):599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E. A., Griffin W. S., Wildenthal K. Isoproterenol-induced cardiomyopathy: changes in cardiac enzymes and protection by methylprednisolone. J Mol Cell Cardiol. 1977 Jul;9(7):565–578. doi: 10.1016/s0022-2828(77)80371-4. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S. Nucleosidediphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci U S A. 1961 Jun 15;47:802–810. doi: 10.1073/pnas.47.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Seabra-Gomes R. Effect of methylprednisolone sodium succinate on hypoxic heart muscle. Cardiovasc Res. 1976 May;10(3):349–358. doi: 10.1093/cvr/10.3.349. [DOI] [PubMed] [Google Scholar]
- Okuda M., Young K. R., Jr, Lefer A. M. Localization of glucocorticoid uptake in normal and ischemic myocardial tissue of isolated perfused cat hearts. Circ Res. 1976 Nov;39(5):640–646. doi: 10.1161/01.res.39.5.640. [DOI] [PubMed] [Google Scholar]
- RAGAN C., HOWES E. L. Effect of cortisone on production of granulation tissue in the rabbit. Proc Soc Exp Biol Med. 1949 Dec;72(3):718-21, illust. doi: 10.3181/00379727-72-17555. [DOI] [PubMed] [Google Scholar]
- Rovetto M. J. Effect of hyaluronidase and methylprednisolone on myocardial function, glucose metabolism, and coronary flow in the isolated ischemic rat heart. Circ Res. 1977 Sep;41(3):373–379. doi: 10.1161/01.res.41.3.373. [DOI] [PubMed] [Google Scholar]
- Spath J. A., Jr, Lane D. L., Lefer A. M. Protective action of methylprednisolone on the myocardium during experimental myocardial ischemia in the cat. Circ Res. 1974 Jul;35(1):44–51. doi: 10.1161/01.res.35.1.44. [DOI] [PubMed] [Google Scholar]
- Topping T. M., Travis D. F. An electron cytochemical study of mechanisms of lysosomal activity in the rat left ventricular mural myocardium. J Ultrastruct Res. 1974 Jan;46(1):1–22. doi: 10.1016/s0022-5320(74)80018-3. [DOI] [PubMed] [Google Scholar]
- Vyden J. K., Nagasawa K., Rabinowitz B., Parmley W. W., Tomoda H., Corday E., Swan H. J. Effects of methylprednisolone administration in acute myocardial infarction. Am J Cardiol. 1974 Nov;34(6):677–686. doi: 10.1016/0002-9149(74)90157-x. [DOI] [PubMed] [Google Scholar]
- Welman E., Peters T. J. Prevention of lysosome disruption in anoxic myocardium by chloroquine and methyl prednisolone. Pharmacol Res Commun. 1977 Jan;9(1):29–38. doi: 10.1016/s0031-6989(77)80051-9. [DOI] [PubMed] [Google Scholar]
- da Luz P. L., Forrester J. S., Wyatt H. L., Diamond G. A., Chag M., Swan H. J. Myocardial reperfusion in acute experimental ischemia. Beneficial effects of prior treatment with steroids. Circulation. 1976 May;53(5):847–852. doi: 10.1161/01.cir.53.5.847. [DOI] [PubMed] [Google Scholar]