Abstract
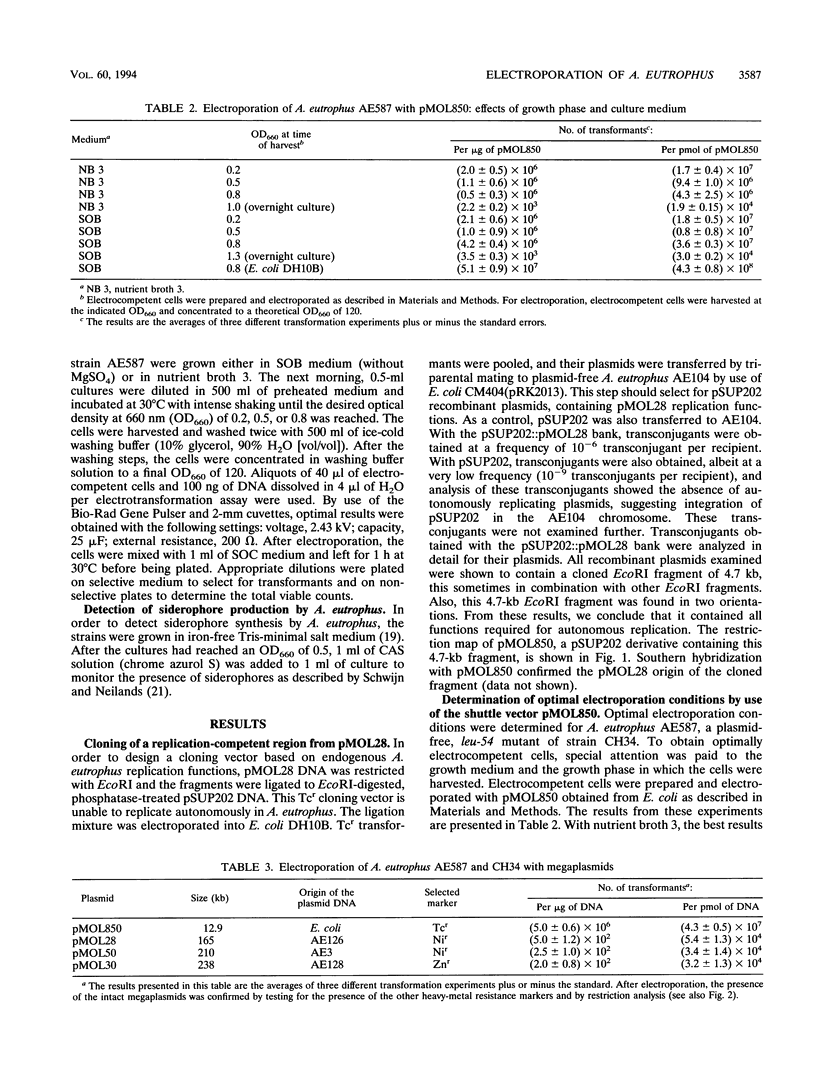
Electroporation was used as a tool to explore the genetics of the heavy-metal-resistant strain Alcaligenes eutrophus CH34. A 12.9-kb A. eutrophus-Escherichia coli shuttle vector, pMOL850, was constructed to optimize electroporation conditions. This vector is derived from the E. coli plasmid pSUP202 and contains the replication region of the A. eutrophus megaplasmid pMOL28. Electroporation was used to transform A. eutrophus CH34 derivatives with megaplasmids (sizes up to 240 kb), and transformants were selected for resistance to heavy metals. Electroporation was also performed with endonuclease-digested genomic DNA. Transformation of markers affecting lysine biosynthesis (lysA194) and biosynthesis of the siderophore alcaligin E were observed. Transfer of the nonselected markers pheB332 and aro-333, linked to lysA194, confirmed the intervention of homologous recombination. However, during transformation of ale::Tn5-Tc, illegitimate recombination and transposition were also observed as an alternative for the inheritance of the Tn5-Tc markers.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bron S., Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res. 1972 May;15(1):1–10. doi: 10.1016/0027-5107(72)90086-3. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. Genetic transformation in Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jan;70(1):84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diels L., Mergeay M. DNA probe-mediated detection of resistant bacteria from soils highly polluted by heavy metals. Appl Environ Microbiol. 1990 May;56(5):1485–1491. doi: 10.1128/aem.56.5.1485-1491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faelen M., Merlin C., Geuskens M., Mergeay M., Toussaint A. Characterization of a temperate phage hosted by Alcaligenes eutrophus strain A5. Res Microbiol. 1993 Oct;144(8):627–631. doi: 10.1016/0923-2508(93)90065-a. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. E., Anderson S. M., Johnston D. M., Cannon R. E. Transformation of Acetobacter xylinum with plasmid DNA by electroporation. Plasmid. 1992 Nov;28(3):194–200. doi: 10.1016/0147-619x(92)90051-b. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune P., Mergeay M., Van Gijsegem F., Faelen M., Gerits J., Toussaint A. Chromosome transfer and R-prime plasmid formation mediated by plasmid pULB113 (RP4::mini-Mu) in Alcaligenes eutrophus CH34 and Pseudomonas fluorescens 6.2. J Bacteriol. 1983 Sep;155(3):1015–1026. doi: 10.1128/jb.155.3.1015-1026.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay M., Gerits J. F'-plasmid transfer from Escherichia coli to Pseudomonas fluorescens. J Bacteriol. 1978 Jul;135(1):18–28. doi: 10.1128/jb.135.1.18-28.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay M., Houba C., Gerits J. Extrachromosomal inheritance controlling resistance to cadmium, cobalt, copper and zinc ions: evidence from curing in a Pseudomonas [proceedings]. Arch Int Physiol Biochim. 1978 May;86(2):440–442. [PubMed] [Google Scholar]
- Mergeay M., Nies D., Schlegel H. G., Gerits J., Charles P., Van Gijsegem F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol. 1985 Apr;162(1):328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersereau M., Pazour G. J., Das A. Efficient transformation of Agrobacterium tumefaciens by electroporation. Gene. 1990 May 31;90(1):149–151. doi: 10.1016/0378-1119(90)90452-w. [DOI] [PubMed] [Google Scholar]
- Nies D., Mergeay M., Friedrich B., Schlegel H. G. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J Bacteriol. 1987 Oct;169(10):4865–4868. doi: 10.1128/jb.169.10.4865-4868.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadouk A., Mergeay M. Chromosome mapping in Alcaligenes eutrophus CH34. Mol Gen Genet. 1993 Aug;240(2):181–187. doi: 10.1007/BF00277055. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shen W. J., Forde B. G. Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res. 1989 Oct 25;17(20):8385–8385. doi: 10.1093/nar/17.20.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M. S., Hooper S. W., Sayler G. S. Plasmid-mediated mineralization of 4-chlorobiphenyl. J Bacteriol. 1985 Sep;163(3):882–889. doi: 10.1128/jb.163.3.882-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. W., Iglewski B. H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989 Dec 25;17(24):10509–10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. F., Smith G. R. Substrate specificity of the DNA unwinding activity of the RecBC enzyme of Escherichia coli. J Mol Biol. 1985 Sep 20;185(2):431–443. doi: 10.1016/0022-2836(85)90414-0. [DOI] [PubMed] [Google Scholar]





