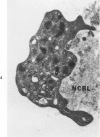
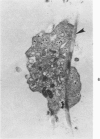
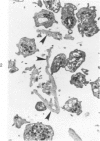
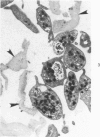
Abstract
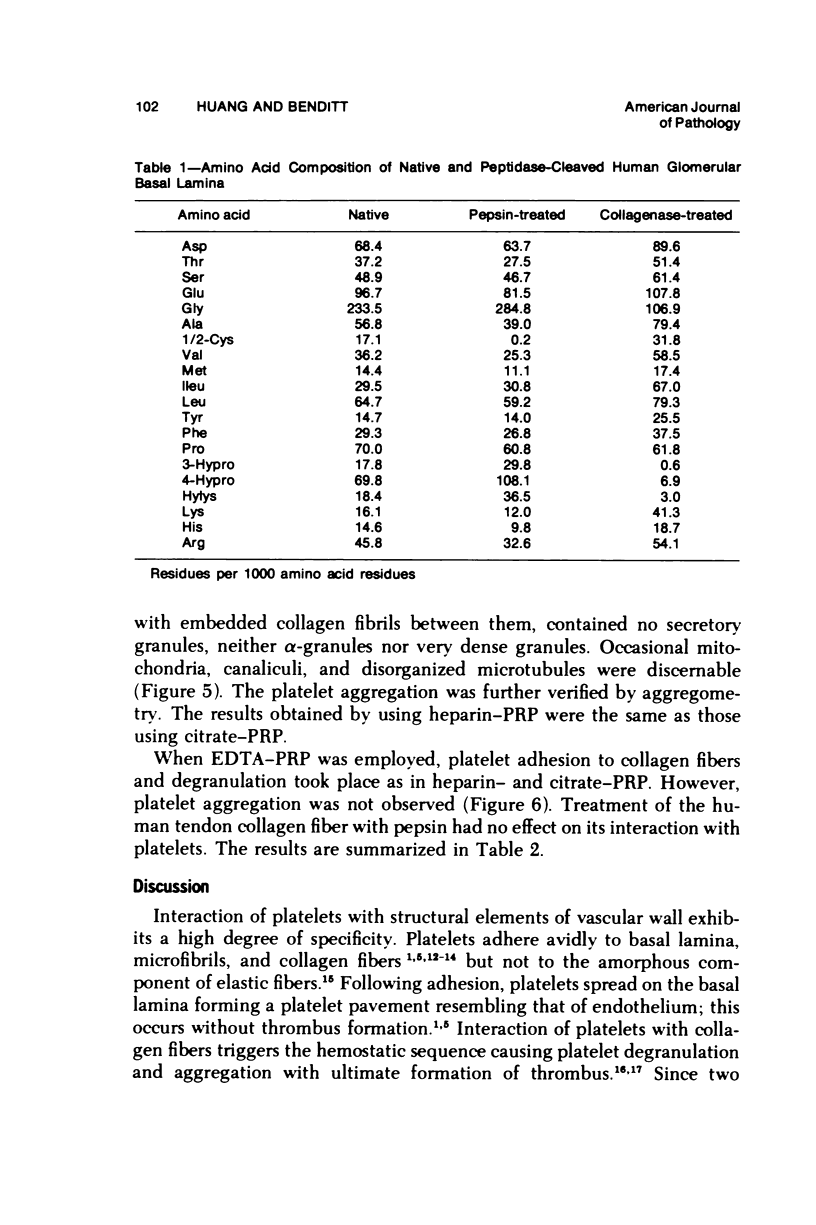
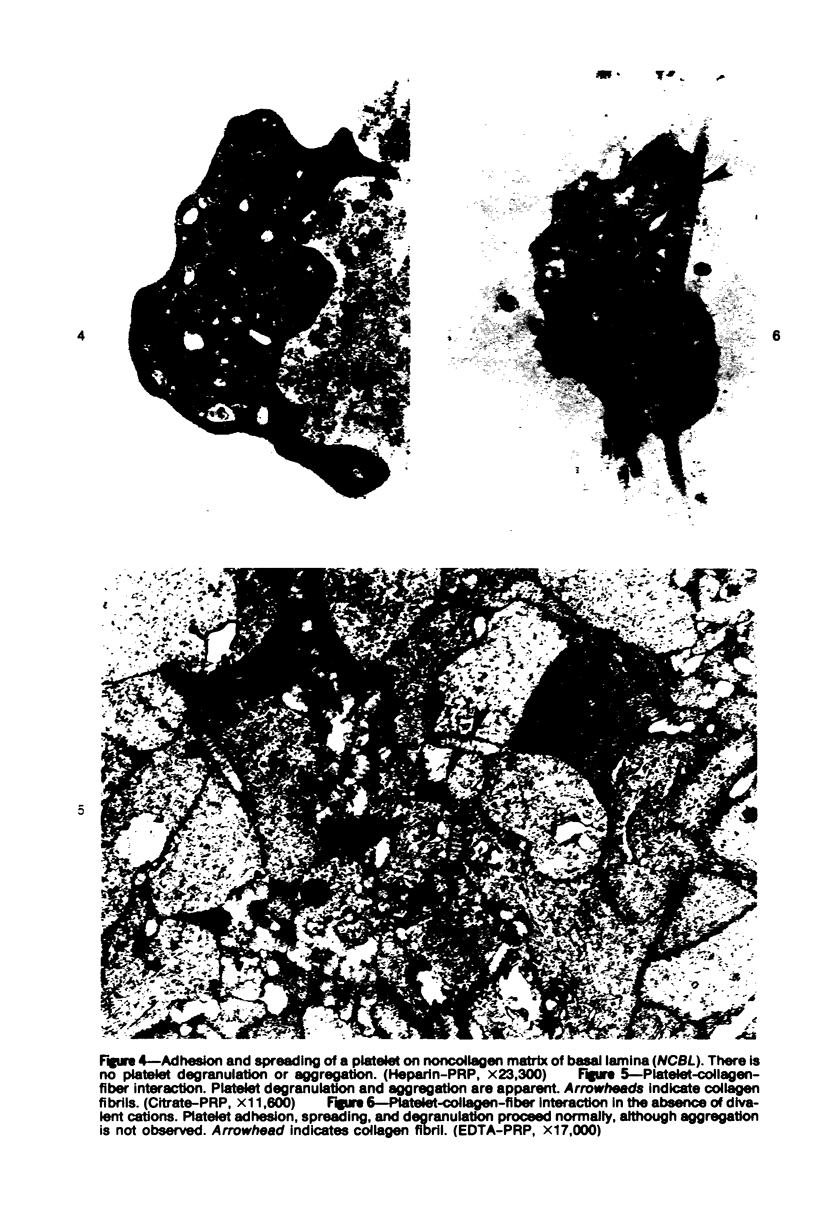
The human glomerular basal lamina (HGBL) is composed of collagenous and noncollagenous glycoproteins. We assessed the role played by each costituent in platelet-basal-lamina interaction by selective cleavage and removal of each component by clostridial collagenase or by pepsin. When noncollagenous proteins are removed from HGBL, human platelets exhibit littel reactivity toward the residual collagen framework of the isolated basal lamina. With the noncollagen matrix of basal lamina, after removal of the bulk of the collagen, platelet adhesion and spreading proceed normally in the presence of divalent cations, similar to what occurs on intact basal lamina. No platelet degranulation or aggregation is observed. The results indicate that the basal lamina collagen, even in its native packing arrangement, lacks affinity for platelet adhesion and is incapable of triggering platelet release reactions. Platelet adhesion and spreading on the basal lamina appears to depend primarily on the presence of the noncollagen components and to require divalent cations. The data suggest the presence on platelets of receptors for basal lamina distinct from those for interstitial collagens. These receptors activate a unique modulation of platelet behavior, ie, adhesion and spreading without degranulation. A difference in biologic function of the basal lamina and interstitial collagens is apparent in these experiments.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner H. R. Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with alpha chymotrypsin-digested subendothelium. Thromb Haemost. 1977 Feb 28;37(1):1–16. [PubMed] [Google Scholar]
- Baumgartner H. R., Stemerman M. B., Spaet T. H. Adhesion of blood platelets to subendothelial surface: distinct from adhesion to collagen. Experientia. 1971 Mar 15;27(3):283–285. doi: 10.1007/BF02138148. [DOI] [PubMed] [Google Scholar]
- Behnke O. Electron microscopical observations on the surface coating of human blood platelets. J Ultrastruct Res. 1968 Jul;24(1):51–69. doi: 10.1016/s0022-5320(68)80016-4. [DOI] [PubMed] [Google Scholar]
- Chesney C. M., Harper E., Colman R. W. Critical role of the carbohydrate side chains of collagen in platelet aggregation. J Clin Invest. 1972 Oct;51(10):2693–2701. doi: 10.1172/JCI107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi F., Palade G. E. Intestinal capillaries. II. Structural effects ofEDTA and histamine. J Cell Biol. 1969 Sep;42(3):706–714. doi: 10.1083/jcb.42.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J. The behavior of collagen units as a model in morphogenesis. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):261–274. doi: 10.1083/jcb.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L., Dingle J. T. Binding of radiolabelled collagen to blood platelets. J Cell Sci. 1974 Oct;16(1):157–166. doi: 10.1242/jcs.16.1.157. [DOI] [PubMed] [Google Scholar]
- Gross J., Highberger J. H., Schmitt F. O. COLLAGEN STRUCTURES CONSIDERED AS STATES OF AGGREGATION OF A KINETIC UNIT. THE TROPOCOLLAGEN PARTICLE. Proc Natl Acad Sci U S A. 1954 Aug;40(8):679–688. doi: 10.1073/pnas.40.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLEM A. J., ODEGAARD A. E. INVESTIGATIONS ON ADENOSINE DIPHOSPHATE (ADP) INDUCED PLATELET ADHESIVNESS IN VITRO. II. STUDIES ON THE MECHANISM. Thromb Diath Haemorrh. 1964 Jul 31;11:305–326. [PubMed] [Google Scholar]
- HELLEM A. J. The adhesiveness of human blood platelets in vitro. Scand J Clin Lab Invest. 1960;12 (Suppl):1–117. [PubMed] [Google Scholar]
- Harker L. A., Hirsh J., Gent M., Genton E. Critical evaluation of platelet-inhibiting drugs in thrombotic disease. Prog Hematol. 1975;9:229–254. [PubMed] [Google Scholar]
- Huang F., Kalant N. Isolation and characterization of antigenic components of rat glomerular basement membrane. Can J Biochem. 1968 Dec;46(12):1523–1532. doi: 10.1139/o68-226. [DOI] [PubMed] [Google Scholar]
- Huang T. W., Lagunoff D., Benditt E. P. Nonaggregative adherence of platelets to basal lamina in vitro. Lab Invest. 1974 Aug;31(2):156–160. [PubMed] [Google Scholar]
- Huang W. Chemical and histochemical studies of human alveolar collagen fibers. Am J Pathol. 1977 Jan;86(1):81–98. [PMC free article] [PubMed] [Google Scholar]
- Jaffe R., Deykin D. Evidence for a structural requirement for the aggregation of platelets by collagen. J Clin Invest. 1974 Mar;53(3):875–883. doi: 10.1172/JCI107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- MacKenzie R. D., Thompson R. J., Gleason E. M. Evaluation of a quantitative platelet-collagen adhesiveness test system. Thromb Res. 1974 Aug;5(2):99–109. doi: 10.1016/0049-3848(74)90061-9. [DOI] [PubMed] [Google Scholar]
- Mason R. G. The interaction of blood hemostitic elements with aritificial surfaces. Prog Hemost Thromb. 1972;1:141–164. [PubMed] [Google Scholar]
- Mitchell W. M., Harrington W. F. Purification and properties of clostridiopeptidase B (Clostripain). J Biol Chem. 1968 Sep 25;243(18):4683–4692. [PubMed] [Google Scholar]
- Nachman R. L., Hubbard A., Ferris B. Iodination of the human platelet membrane. Studies of the major surface glycoprotein. J Biol Chem. 1973 Apr 25;248(8):2928–2936. [PubMed] [Google Scholar]
- Page R. C., Jones D., Hansen R. An accelerated step-gradient procedure for the automatic analysis of collagen hydrolyzates. Anal Biochem. 1970 Oct;37(2):293–297. doi: 10.1016/0003-2697(70)90051-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard B. L. Platelet adhesion in the rabbit abdominal aorta following the removal of endothelium with EDTA. Proc R Soc Lond B Biol Sci. 1972 Jul 25;182(1066):103–108. doi: 10.1098/rspb.1972.0069. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Stemerman M. B. Platelet adhesion. Ann N Y Acad Sci. 1972 Oct 27;201:13–21. doi: 10.1111/j.1749-6632.1972.tb16284.x. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Baumgartner H. R., Spaet T. H. The subendothelial microfibril and platelet adhesion. Lab Invest. 1971 Mar;24(3):179–186. [PubMed] [Google Scholar]
- Stemerman M. B., Spaet T. H. The subendothelium and thrombogenesis. Bull N Y Acad Med. 1972 Feb;48(2):289–301. [PMC free article] [PubMed] [Google Scholar]
- Suresh A. D., Stemerman M. B., Spaet T. H. Rabbit heart valve basement membrane: low platelet reactivity. Blood. 1973 Mar;41(3):359–367. [PubMed] [Google Scholar]
- Thompson P. L., Papadimitriou J. M., Walters M. N. Suppression of leucocytic sticking and emigration by chelation of calcium. J Pathol Bacteriol. 1967 Oct;94(2):389–396. doi: 10.1002/path.1700940219. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Baumgartner H. R. Filling gaps in the vascular endothelium with blood platelets. Nature. 1967 Dec 16;216(5120):1126–1128. doi: 10.1038/2161126a0. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Lawley K. R. Isolation and initial characterization of human basement membrane collagens. Biochem Biophys Res Commun. 1976 May 23;76(2):376–384. doi: 10.1016/0006-291x(77)90735-5. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Vracko R., Benditt E. P. Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J Cell Biol. 1972 Nov;55(2):406–419. doi: 10.1083/jcb.55.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J. The pharmacology of platelet inhibition. Prog Hemost Thromb. 1972;1:199–231. [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. H., Mason R. G. Ultrastructural aspects of interactions of platelets with microcrystalline collagen. Am J Pathol. 1976 Jan;82(1):129–142. [PMC free article] [PubMed] [Google Scholar]