Abstract
SAMMA, a mandelic acid condensation polymer, exhibits a broad antimicrobial activity against several sexually transmitted pathogens including HIV. Here we demonstrated that SAMMA suppressed HIV transmission by dendritic cells (DCs), one of the first target cells for primary infection. The greatest inhibitory effect was achieved when SAMMA was present during the co-culture with target cells. The inhibitory effect of SAMMA on DC-mediated HIV transmission was not due to cytotoxicity. Analysis of the level of DC-associated HIV p24 antigen revealed that SAMMA prevented HIV internalization by DCs when the virus was pre-incubated with the compound. In contrast, pre-incubation of DCs with SAMMA followed by wash-off did not affect the amount of cell-associated HIV p24 antigen. In addition, SAMMA blocked HIV glycoprotein-mediated cell-cell fusion. This study suggests that SAMMA prevents HIV infection through multiple mechanisms.
Keywords: Microbicide, Dendritic cell, HIV transmission, cell-cell fusion, non-sulfonated polyanions
1. Introduction
Sexual transmission is the most common route of acquiring human immunodeficiency virus (HIV). Women account for nearly half of those infected worldwide and more than 70% in sub-Saharan Africa (UNAIDS report 2006). Women are more susceptible to HIV because of a higher prevalence of sexual transmitted infections (STIs) in addition to hormone changes [1,2]. As an effective vaccine against HIV infection is unlikely to be available in the near future, development of a safe and effective topical microbicide to prevent sexual transmission of HIV as well as other STIs becomes an urgent need. Indeed, studies in rhesus macaques support the concept that topical microbicides can prevent vaginal virus transmission [3–5].
Dendritic cells (DCs) present in the vaginal epithelium and lamina propria, are one of the first target cells for primary infection with simian immunodeficiency virus (SIV) and HIV [6–8]. DCs can capture HIV and present it to CD4+ T cells efficiently in vitro, suggesting that genital mucosa DCs may uptake and transmit HIV to the surrounding CD4+ T cells or to the draining lymph nodes [6–11]. HIV can establish infection by binding to CD4, mannose-binding C-type lectin receptor (MCLR), other MCLR like mannose receptor, and non-MCLRs on the surface of different subsets of DCs [12–16]. Therefore, it would be an important feature for an ideal topical microbicide to block DC-mediated HIV transmission.
SAMMA, a mandelic acid condensation polymer, has been shown to be a potential candidate as topical microbicide. SAMMA, a nonsulfonated polyanion, differs from N-9 (a detergent) or cellular sulfate (sulfonate polyanion), two microbicide candidates in clinical trials that not only failed to prevent but enhanced HIV transmission in women [17–19] and aidsinfo.nih.gov). It exhibits potent anti-HIV activity in vitro by interacting with HIV gp120 at a high affinity and subsequently blocking HIV entry [20,21]. Importantly, it also inhibits other sexually transmitted pathogens including herpes simplex viruses (HSV) 1 and 2, Clamydia trachomatis, and Neisseria gonorrhoeae [20,22] that are associated with HIV enhancement [23–27]. In addition, SAMMA does not cause cytotoxicity in mammalian cells or affect the growth of commensal bacteria such as lactobacilli [22].
Here we evaluated the effect of SAMMA on DC-mediated HIV transmission. We demonstrated that SAMMA inhibited HIV capture and transfer by DCs as well as HIV glycoprotein-mediated cell-cell fusion. This study demonstrates that SAMMA can block HIV infection by interfering with HIV interaction with not only CD4 but also other receptors on various target cells, suggesting utilization of multiple mechanisms for HIV prevention.
2. Materials and Methods
2.1 DC preparation and cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient centrifugation. CD14+ monocytes were positively selected from PBMCs using CD14+ magnetic beads from Miltenyi Biotec, Inc (Auburn, CA). Monocyte derived dendritic cells (MDDCs) were obtained by culturing CD14+ monocytes in the presence of IL-4 (100U/ml, R&D Systems) and GM-CSF (1000U/ml, R&D systems) for 6–7 days and cytokines were added every other day. This DC population has a CD14−, CD11c+, HLA-DR+, CD3−, and CD83− phenotype. Autologus CD4+ T cells were treated with phytohemagglutinin (PHA) at 5 μg/ml for 48–72 hr and subsequently cultured in complete RPMI with 10% fetal bovine serum (FBS) and supplemented with IL-2 at a concentration of 20–50 units/ml.
2.2 DC-mediated HIV transmission
To study DC-mediated HIV transmission, DCs were exposed to R5 strain HIVBaL or pseudotyped HIVJR-FL luciferase reporter virus in the presence or absence of SAMMA. Peseudotyped HIVJR-FL luciferase reporter virus was prepared as described previously [28]. After washing off, DCs were co-cultured with susceptible cells including PHA-activated primary CD4+ cells or HeLa-CD4.CCR5 cells. HIV p24 levels in the media were measured by ELISA (SAIC-Frederick Inc) for a multiple-round infection assay, whereas luciferase activity in target cells was determined for a single-round infection assay as described previously [29].
2.3 Cytotoxicity assay
DCs, activated primary CD4+ T cells, or TZM-bl cells at 2–5×104 in a 96-well plate were treated with SAMMA at indicated concentrations for 2 hr or three days at 37°C. Cytotoxicity was determined by measuring the number of viable cells by the MTS assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay, Promega, Inc) according to manufacture’s instructions. MTS substrate was added to cells and cells were incubated at 37°C for 1 hr. The conversion of MTS into soluble formazan by dehydrogenase enzymes found in metabolically active cells was measured by quantifying of formazan products using absorbance at 490 nm in a microplate reader.
2.4 HIV capture
HIV-1 capture assay was performed as described elsewhere with modifications [30]. MDDCs were seeded at 2×105 per well in 96-well flat-bottom plates. HIV-1BaL (3 ng per well) was pre-incubated in the absence or presence of SAMMA for 1h at 37°C. DCs were then incubated with virus for 2 hr at 4°C (for binding) or 37°C (for binding and internalization). Cells were washed four times to remove unbound virus and SAMMA and lysed with 1% Triton X-100. Cell-associated HIV p24 antigen was measured by p24 ELISA (NCI, Frederick).
To determine whether SAMMA interacted with cellular receptors on DCs and subsequently affected HIV capture, DCs were pre-incubated with SAMMA at 37°C for 1 hr, washed four times with PBS before HIV exposure at 4°C or 37°C for 2 hr. Cells were washed four times and the level of cell-associated HIV p24 antigen was measured by ELISA.
2.5 HIV glycoprotein-mediated cell-cell fusion
HeLa cells expressing Tat protein (HeLa-Tat) were transfected with the HIVJR-FL envelope plasmid (gift of D. Littman, New York Univeristy) for 48 hr. HeLa-Tat cells at 5×105 expressing HIV glycorpoteins were treated with SAMMA or T-20 (from ARRRP) at 37°C for 1 hr before addition to TZM-bl indicator cells (from ARRRP; gift of Drs J.C. Kappes, and X. Wu), which express CD4, CXCR4 and CCR5 co-receptors. TZM-bl cells, seeded at 5×105 per well grown in a 48-well plate, contain HIV long terminal repeat (LTR) driven beta galactosidase and luciferase reporter genes. When cell-cell fusion occurs, Tat proteins activate these reporter genes. After incubation for 8–24 hr at 37°C, TZM-bl cells were treated with lysis buffer (Promega Corp.) and luciferase activity (in releative lifght units [RLUs]) was measured using Perkin-Elmer 1420 Luminometer.
3. Results
3.1 SAMMA blocked HIV transmission by DCs
Both intraepithelial and submucosal DCs and CD4+ T cells are the primary target cell populations for SIV and HIV-1 (reviewed in [6,8]). It has been shown that SAMMA at 100 μg/ml inhibits HIV infection (greater than 90%) in primary CD4+ T cells [20]. Here we investigated the effect of SAMMA on HIV-1 transmission by DCs. Human monocyte derived DCs (MDDCs) were exposed to replication-competent R5 virus HIVBaL in the presence or absence of SAMMA at 100 μg/ml for 2 hr. An HIV-1 R5 strain was used as they are preferentially transmitted in primary infection [31]. DCs were washed and then co-cultured with autologous PHA-activated primary CD4+ T cells in the presence or absence of SAMMA at 100 μg/ml. HIV-1 infection was monitored by measuring the level of HIV p24 antigen in the media by ELISA. DCs in the absence of CD4+ T cells produced little virus (~ 7 ng/ml at days 9 and 14 after infection, data not shown) in comparison to DC-T cell co-culture that produced 800–1200 ng/ml (Fig. 1a, control). SAMMA blocked DC-mediated HIV transmission by greater than 99% when it was present during the all co-culture period (Fig 1a).
Fig. 1.
SAMMA inhibits HIV transmission by DCs. (a) MDDCs (1.25–2×105 per well) were exposed to replication-competent R5 virus HIVBaL at MOI of 0.002 in the presence or absence of SAMMA at 100 μg/ml at 37°C for 2 hr. Cells were washed and co-cultured with autologus activated primary CD4+ T cells (1×106) in complete media in the presence or absence of SAMMA at 100 μg/ml. (b) HIVBaL was pre-incubated without (control) or with SAMMA at 100 μg/ml at 37°C for 1 hr followed by addition to DCs (2×105) for 2 hr in the absence or presence of the inhibitor. After washing four times with media, DCs (1.25 × 105) were incubated with activated primary CD4+ T cells (1 × 106) in the absence of SAMMA. HIV released into media was monitored by measuring the level of HIV p24 antigen at day 0, 5, 7, 9, 14 after infection. Data in Fig. 1a are mean ± SD of duplicated samples and represent two independent experiments. In the wash-off setting (panel b), results from two different donors are shown.
Once immature DCs capture HIV in the vaginal mucosa, they can transfer virus to neighboring CD4+ T cells or migrate to lymphatic tissue followed by HIV transmission to CD4+ T cells. An experiment was set up to simulate a possible in vivo scenario when SAMMA would be present only during HIV capture by DCs but absent during HIV transfer to CD4+ T in draining lymph nodes. HIV-1BaL was first exposed to SAMMA for 1 hr followed by incubation with DCs for 2 hr to allow HIV capture. DCs were then washed extensively before co-culturing with activated primary CD4+ T cells. In wash-off settings, SAMMA at 100 μg/ml inhibited HIV transmission by ~50–90% at day 9 after infection depending on the donors (Fig. 1b). In addition, we observed the lack of increase in the level of p24 at day 14 after infection, suggesting residual SAMMA and or persistent effect of SAMMA on the cell after washing off. Taken together, results in Fig. 1 suggest that SAMMA can block HIV transmission in vaginal/cervical mucosa but its maximal effect is when it is present both for DC capture and T cell transfer.
To determine the effect of SAMMA on HIV transmission in trans, we used a replication-defective R5 strain HIV JR-FL pseudotyped virus containing a luciferase reporter gene to measure HIV transfer by DCs to target cells. Expression of luciferase provides a quantitative measure of a single-round of HIV infection [32]. MDDCs, capturing HIV primarily through MCLRs [33], were incubated with SAMMA, anti-DC-SIGN antibody, or mannose receptor ligand mannan, which blocks gp120 binding to C-type lectins [34], for 1 hr followed by exposure to viruses for 2 hr in the absence or presence of inhibitors or antibodies (Fig. 2a). Although CD4 is not the predominant receptor on MDDCs for the binding of HIV gp120 [33], DCs were also treated with anti-CD4 antibody in comparison to SAMMA (Fig. 2b). After 2hr incubation with HIV, DCs were extensively washed then added to HeLa cells expressing CD4 and CCR5, which provided a better luciferase read-out in comparison to primary CD4+ T cells. After incubation for 48 hr, DCs were removed by washing and HIV transfer to HeLa-CD4.CCR5 cells was determined by measuring luciferase activity in the cells. SAMMA at 100 μg/ml effectively inhibited HIV transmission by DCs when it was present only during exposure of DCs to virus in a single-cycle infection assay (Figs. 2a and 2b). As expected, mannan inhibited the ability of DCs to transmit HIV. In agreement with previous reports [30] [33,35], anti-DC-SIGN antibody partially blocked HIV transmission by DCs, although it completely blocked HIV transmission by a cell line expressing DC-SIGN by greater than 95% (data not shown). No effect was observed in samples treated with isotyped control antibodies (Fig. 2b). Anti-CD4 antibody (Leu3a) at 10 μg/ml, which inhibited HIV infection in activated primary CD4+ T cells (data not shown), did not block DC-mediated HIV transmission when it was present during HIV capture by DCs (Fig. 2b).
Fig. 2.
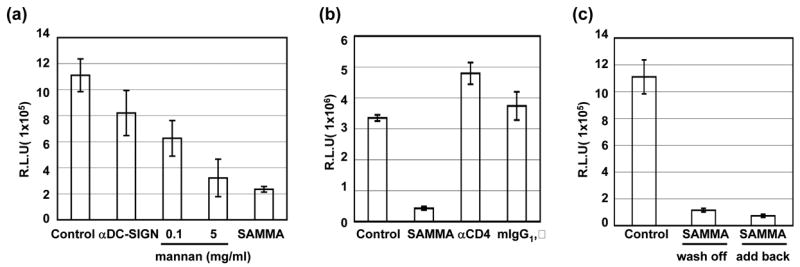
SAMMA inhibits HIV infection in trans by DCs in a single-round infection assay. (a) MDDCs (1×106) were incubated with SAMMA (10 and 100 μg/ml), mannan (0.1 and 5 mg/ml) or anti-DC-SIGN antibody (20 μg/ml, mAb 612 from R&D systems) for 1 hr at 37°C and then exposed to pseudotyped HIVJR-FL luciferase reporter virus for 2 hr at 37°C. Cells were washed four times before co-culturing with HeLa-CD4.CCR5 cells for 48 hr. DCs were removed by washing and luciferase activity in relative light unit (R.L.U.) in HeLa cells were measured. (b). DCs were incubated with SAMMA at 100 μg/ml, anti-CD4 antibody (10 μg/ml, Leu3a from BD Biosciences) or an isotype control antibody (mouse IgG1, κ) at 37°C for 1 hr followed by exposure to pseudotyped HIVJR-FL luciferase reporter virus for 2 hr. DCs were washed and then co-cultured with HeLa-CD4.CCR5 cells for 48 hr before measurement of luciferase activity in HeLa cells. (c) DCs were exposed to pseudotyped HIVJR-FL luciferase reporter virus for 2 hr at 37°C and then incubated without or with SAMMA at 100 μg/ml for 1 hr at 37°C. DCs were washed four times with PBS and then co-cultured with HeLa-CD4.CCR5 cells in the absence (wash off) or presence (add back) of SAMMA for 48 hr. Luciferase activity in HeLa cells was measured. Data are mean ± SD of triplicated samples and represent three independent experiments.
We then assessed whether SAMMA could inhibit HIV transmission when added after DCs were exposed to HIV. MDDCs were exposed to HIV followed by incubation with SAMMA. The inhibitor was washed away before co-culture of DCs with target cells. SAMMA blocked HIV transmission in the wash-off setting (Fig. 2c). The inhibitory effect of SAMMA on HIV transmission by HIV-exposed DCs was similar when the inhibitor was present during the co-culture (add back) in a single-cycle infection assay.
3.2 SAMMA is not cytotoxic to DCs
To ensure that the inhibitory effect of SAMMA on DC-mediated HIV transmission was not due to cytotoxicity, we examined viability of DCs and CD4+ T cells in the presence of SAMMA by MTS assay. SAMMA had no effect on the viability of DCs or CD4+ T cells when present in the culture for three days at the dose up to 500 μg/ml, indicating that inhibition of DC-mediated HIV transmission was not associated with cytotoxicity (Fig. 3).
Fig. 3.
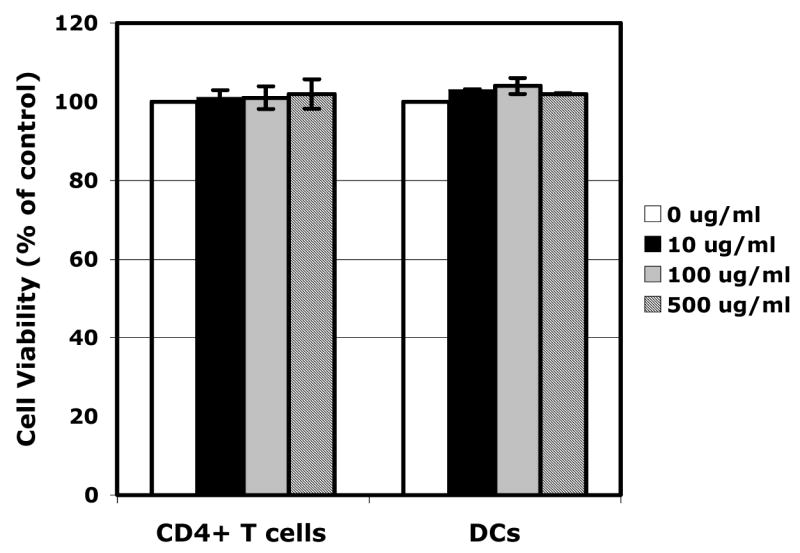
SAMMA does not cause cytotoxicity in DCs and primary CD4+ T cells. DCs or activated primary CD4+ T cells (5×104) were treated with SAMMA at 10, 100, 500 μg/ml for three days at 37°C. Cytotoxicity was determined by measuring the number of viable cells by the MTS assay (Promega). MTS substrate was added to cells for 1 hr at 37°C. Data are mean ± SD of triplicated samples and represent two independent experiments.
3.3 SAMMA blocked DC-mediated HIV capture
The ability of SAMMA to block DC-mediated HIV transmission when it was present only during the initial exposure of DCs to HIV suggests that SAMMA may interfere with HIV capture by DCs. To determine the effect of SAMMA on HIV capture by DCs, HIV-1BaL was incubated in the absence (control) or presence of SAMMA at 100 μg/ml for 1 hr at 37°C before exposure to DCs for 2 hr at 37°C (for viral binding and internalization, Fig. 4a) or 4°C (viral binding only, Fig. 4b). DCs were then washed extensively, lysed and cell-associated HIV p24 antigen was measured. SAMMA at 100 μg/ml blocked DC-mediated HIV capture by ~58 to 99% depending on the DC donors (Fig. 4a), which correlated with the effect of SAMMA on DC-mediated HIV transmission when SAMMA was only present during HIV capture (Fig. 1b). When DCs were exposed to HIV-1BaL at 4°C in the absence and presence of SAMMA, no significant difference was detected, indicating that SAMMA did not affect HIV bindings to DCs (Fig. 4b). Thus, results in Figs. 4a and 4b suggested that SAMMA interfered with internalization of HIV by DCs.
Fig. 4.
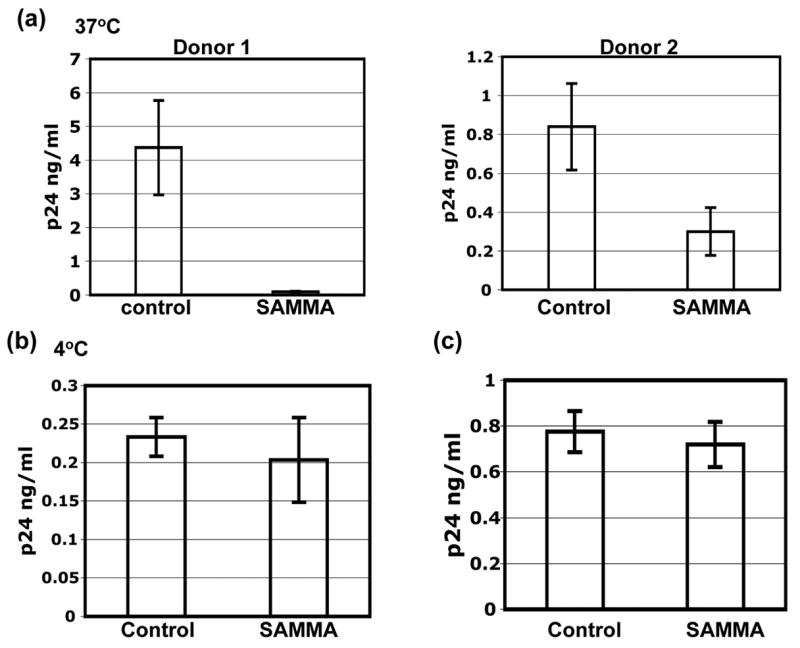
SAMMA inhibits HIV capture by DCs. MDDC were seeded at 2×105 per well in 96-well flat-bottom plates. HIV-1BaL (3 ng per well) was pre-incubated in the absence (control) or presence of SAMMA for 1hr at 37°C. DCs were then incubated with virus for 2 hr at 37°C (a) or 4°C (b) in the absence (control) or presence of the inhibitor. Cells were washed four times to remove unbound virus and SAMMA and lysed with 1% Triton X-100. Cell-associated HIV p24 antigens were measured by p24 ELISA (NCI, Frederick). Data are mean ± SD of duplicated samples. In Fig. 4a, results from two donors are shown. In Figs. 4b and 4c, results represent two independent experiments from two DC different donors. (c) DCs were treated without (control) or with SAMMA at 100 μg/ml at 37°C for 1 hr and washed four times with PBS followed HIV exposure for 2 hr. HIV-exposed DCs were washed and lysed. Cell-associated HIV p24 antigen was measured by ELISA. Results represent two independent experiments.
To delineate whether SAMMA blocked DC-mediated HIV capture by acting on the cell, DCs were pre-incubated with SAMMA for 1 hr at 37°C and washed four times before exposure to HIV-1BaL for 2 hr at 4°C (for binding) or 37°C (for binding and internalization). DCs were then washed four times with PBS and lysed. Cell-associated HIV p24 antigen was measured by ELISA. When DCs were pre-treated with SAMMA and washed before HIV exposure, SAMMA did not block HIV binding or binding/internalization by DCs (data not shown and Fig. 4c), suggesting that inhibition of DC-mediated HIV capture is primarily due to the effect on the virus.
3.4 SAMMA blocks HIV glycorprotein-mediated cell-cell fusion
SAMMA effectively block DC-mediated HIV transmission when the inhibitor was present during the co-culture. DC-mediated HIV transmission to CD4+ cells occurs at a cell-cell junction referred to as the infectious synapse [36–38]. The potent anti-HIV activity of SAMMA during the co-culture led us to investigate the effect of SAMMA on HIV glycoprotein-mediated cell-cell fusion. HeLa cells stably expressing Tat protein (HeLa-Tat cells) were transiently transfected with a vector expressing HIVJR-FL envelope glycoprotein for 48 hr. HeLa-Tat cells expressing HIV glycoproteins were then incubated with SAMMA at 10, 100, and 1000 μg/ml for 1 hr at 37°C before addition to TZM cells, indicator cells that express CD4 and CXCR4 and CCR5 co-receptors and contain an HIV long terminal repeat (LTR)-driven reporter gene. HIV Tat protein synthesized in cells expressing HIV glycoproteins activate the HIV LTR in the indicator cells after cell-cell fusion occurs. The fusion inhibitor, T-20, at 100 nM was included to ensure the specificity of this assay. As expected, T-20, which blocks HIV trans infection of target cells by HIV-exposed MDDCs [39], inhibited HIV glycoprotein-mediated cell-cell fusion. SAMMA at 10 μg/ml inhibited HIV glycoprotein-mediated cell-cell fusion by 47%. A greater inhibitory effect was achieved with SAMMA at 100 μg/ml (~98%) and 1000 μg/ml (>99%) (Fig. 5a). SAMMA also blocked cell-cell fusion when the donor cells expressed HIV X4 envelope proteins (data not shown).
Fig. 5.
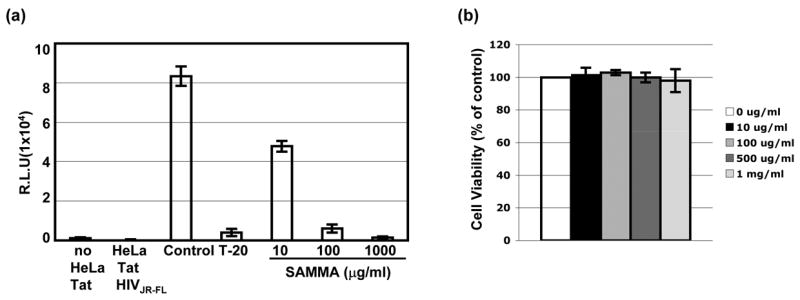
SAMMA inhibits HIV glycoprotein-mediated cell-cell fusion. HeLa-Tat cells were transfected HIVJR-FL envelope plasmid for 48 hr. Cells were then treated with a fusion inhibitor T-20 or SAMMA at 37°C for 1 hr before addition to TZM cells, indicator cells expressing CD4, CXCR4 and CCR5 and containing a luciferase reporter gene driven by HIV LTR. After incubation at 37°C for 24 hr, luciferase activity was measured. Data are mean ± SD of duplicated samples and represent three independent experiments. (b) Viability of TZM-bl cells in the presence of SAMMA at various concentrations for 48 hr was determined by MTS assay as described in Fig. 3.
To ensure inhibition of HIV glycoprotein-mediated cell-cell fusion by SAMMA was not due to cytotoxicity, HeLa-Tat or TZM-bl cells were treated with SAMMA at various concentrations for 24 hr. Cell viability was determined by MTS assay. No significant difference in cell viability between cells in the absence or presence of SAMMA at up to 1000 μg/ml (Fig. 5b and data not shown), indicating that the inhibitory effect on cell-cell fusion in Fig. 5a was not due to cytotoxicity.
4. Discussion
SAMMA has been shown to be a potential candidate for topical microbicides because of its broad antimicrobial and antiviral activities against Chlamydia trachomatis, Neisseria gonorrhoeae, HIV and HSV [20–22]. Importantly, it does not cause cytotoxicity in many cell lines and primary cells including human CD4+ T cells and macrophages (Fig. 3, [20]). Here we investigated the effect of SAMMA on DC-mediated HIV transmission, which is an important initial step in sexual transmission of HIV. SAMMA is not cytotoxic for DCs and primary activated CD4+ T cells when present in the culture for 3 days. SAMMA effectively blocked HIV transmission by DCs when present during co-culture period. In addition, SAMMA blocked HIV transmission when it was incubated with HIV-exposed DCs followed by wash-off before co-culture with target cells, suggesting its inhibitory effect persists. The observation that SAMMA suppressed HIV glycoprotein-mediated cell-cell fusion by greater than 95% suggests one possible mechanism by which this compound inhibits HIV transfer when present during the co-culture period. SAMMA is likely to inhibit glycoprotein-mediated cell-cell fusion through interfering with the interaction between HIV glycoprotein and CD4 receptors, although it remains to be determined whether SAMMA can block the step of viral fusion.
We demonstrated that SAMMA blocked DC-mediated HIV-1 transmission by preventing HIV capture by DCs (Fig. 4). Specifically, SAMMA affected the step of HIV internalization [8]. Further studies using specific inhibitors targeting specific steps such endocytosis or fusion are required to dissect in detail the mechanism by which SAMMA inhibits DC-mediated HIV capture. We demonstrated that SAMMA has a similar inhibitory profile to mannan and greater than anti-DC-SIGN or anti-CD4 antibodies (Figs. 2a and 2b), suggesting that inhibition of capture followed by transfer may be mediated by blocking HIV interactions with other MCLRs rather than DC-SIGN or CD4. Macrophage mannose-receptors and an unidentified trypsin-resistant CLR have been reported to contribute to gp120 binding in MDDCs [33]. It remains to be determined whether SAMMA interferes with HIV capture by these receptors on the surface of DCs. In addition, the varying abundance of specific receptors among different donors may contribute to differential inhibitory effects of SAMMA on DC-mediated HIV capture and transmission in wash-off settings (Figs 1b and 4a). The incomplete block of trans infection when SAMMA is present only during DC incubation as well as variation in effectiveness with different donor DC supports the use of SAMMA in combination rather than alone. Nevertheless, our study and previous reports suggest that several mechanisms are involved in the anti-HIV effect of SAMMA [20,21].
SAMMA also inhibited HIV transfer from HIV-exposed DCs to the indicator cells when added following HIV capture and washed off before co-culture. The sustained effect on HIV transfer from HIV-exposed DCs after washing off has been reported previously with the inhibitors such as CD4-IgG2 and antimicrobial peptides, although the mechanism is not clear [39,40]. It is of note that PRO2000, a sulfated anionic polymer in phase III clinical trials for topical microbicides against HIV, exhibits a similar inhibitory effect on HIV transmission from HIV-exposed DCs in the wash-off setting (Natalia Teleshova, manuscript submitted). Like CD4-IgG2, PRO2000 and SAMMA bind to HIV gp120 [21]. Presumably, residual SAMMA and or persistent effects of SAMMA on HIV-exposed cells inactivates HIV or alters the formation of virological synapse [37,38], resulting in the inhibition of HIV transmission.
In conclusion, our study demonstrates that SAMMA inhibited DC-mediated HIV transmission through blocking DC-mediated HIV capture and subsequent transfer. SAMMA also efficiently inhibited HIV glycoprotein-mediated cell-cell fusion, supporting its potential as an anti-HIV topical microbicide in combination with other inhibitors.
Acknowledgments
We thank Irini Scordi-Bello for initiating the project and Arevik Mosoian for discussions. This work was supported by NIH grant P01 HD41763 to M.E.K.
Abbreviations
- HIV
Human immunodeficiency virus
- DC
dendritic cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308:1582–3. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 2.Simon V, Ho DD, Abdool Karim Q. HIV/AIDS epidemiology, athogenesis, prevention, and treatment. Lancet. 2006;368:489–504. doi: 10.1016/S0140-6736(06)69157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 4.Veazey RS, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–6. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 5.Lederman MM, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 6.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–52. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–95. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–68. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–7. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 10.Pope M, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–98. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 11.Pinchuk LM, Polacino PS, Agy MB, Klaus SJ, Clark EA. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity. 1994;1:317–25. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee B, et al. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol. 2001;75:12028–38. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turville SG, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–9. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 15.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–83. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 16.Gummuluru S, Rogel M, Stamatatos L, Emerman M. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J Virol. 2003;77:12865–74. doi: 10.1128/JVI.77.23.12865-12874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Damme L, Chandeying V, Ramjee G, Rees H, Sirivongrangson P, Laga M, Perriens J. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. Aids. 2000;14:85–8. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 18.Van Damme L, et al. Safety evaluation of nonoxynol-9 gel in women at low risk of HIV infection. Aids. 1998;12:433–7. doi: 10.1097/00002030-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Van Damme L, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 20.Herold BC, et al. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J Virol. 2002;76:11236–44. doi: 10.1128/JVI.76.22.11236-11244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scordi-Bello IA, et al. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005;49:3607–15. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaneveld LJ, et al. Use of mandelic acid condensation polymer (SAMMA), a new antimicrobial contraceptive agent, for vaginal prophylaxis. Fertil Steril. 2002;78:1107–15. doi: 10.1016/s0015-0282(02)04210-3. [DOI] [PubMed] [Google Scholar]
- 23.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 24.Plummer FA. Heterosexual transmission of human immunodeficiency virus type 1 (HIV): interactions of conventional sexually transmitted diseases, hormonal contraception and HIV-1. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S5–10. [PubMed] [Google Scholar]
- 25.Cohen MS, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 26.Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J Acquir Immune Defic Syndr. 2000;24:48–56. doi: 10.1097/00126334-200005010-00009. [DOI] [PubMed] [Google Scholar]
- 27.Mabey D. Interactions between HIV infection and other sexually transmitted diseases. Trop Med Int Health. 2000;5:A32–6. doi: 10.1046/j.1365-3156.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang TL, Vargas J, Jr, DelPortillo A, Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest. 2005;115:765–73. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang TL, Mosoian A, Pine R, Klotman ME, Moore JP. A soluble factor(s) secreted from CD8(+) T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. J Virol. 2002;76:569–81. doi: 10.1128/JVI.76.2.569-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trumpfheller C, Park CG, Finke J, Steinman RM, Granelli-Piperno A. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int Immunol. 2003;15:289–98. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- 31.Meng G, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–6. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 32.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–44. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 33.Turville SG, Arthos J, Donald KM, Lynch G, Naif H, Clark G, Hart D, Cunningham AL. HIV gp120 receptors on human dendritic cells. Blood. 2001;98:2482–8. doi: 10.1182/blood.v98.8.2482. [DOI] [PubMed] [Google Scholar]
- 34.Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1992;89:8356–60. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol. 2007;81:2519–23. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–7. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 37.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–10. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–88. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ketas TJ, et al. Human immunodeficiency virus type 1 attachment, coreceptor, and fusion inhibitors are active against both direct and trans infection of primary cells. J Virol. 2003;77:2762–7. doi: 10.1128/JVI.77.4.2762-2767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanCompernolle SE, et al. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J Virol. 2005;79:11598–606. doi: 10.1128/JVI.79.18.11598-11606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]



