Abstract
Background
Consuming foods low in energy density (kcal/g) decreases energy intake over several days, but the effectiveness of this strategy for weight loss has not been tested.
Objective
The effects on weight loss of 2 strategies for reducing the energy density of the diet were compared over 1 y.
Design
Obese women (n = 97) were randomly assigned to groups counseled either to reduce their fat intake (RF group) or to reduce their fat intake and increase their intake of water-rich foods, particularly fruit and vegetables (RF+FV group). No goals for energy or fat intake were assigned; the subjects were instructed to eat ad libitum amounts of food while following the principles of their diet.
Results
After 1 y, study completers (n = 71) in both groups had significant decreases in body weight (P < 0.0001). Subjects in the RF+FV group, however, had a significantly different pattern of weight loss (P = 0.002) than did subjects in the RF group. After 1 y, the RF+FV group lost 7.9 ± 0.9 kg and the RF group lost 6.4 ± 0.9 kg. Analysis of all randomly assigned subjects also showed a different pattern of weight loss between groups (P = 0.021). Diet records indicated that both groups had similar reductions in fat intake. The RF+FV group, however, had a lower dietary energy density than did the RF group (P = 0.019) as the result of consuming a greater weight of food (P = 0.025), especially fruit and vegetables (P = 0.037). The RF+FV group also reported less hunger (P = 0.003).
Conclusion
Reducing dietary energy density, particularly by combining increased fruit and vegetable intakes with decreased fat intake, is an effective strategy for managing body weight while controlling hunger.
Keywords: Energy density, fruit and vegetables, water-rich foods, fat intake, obesity, weight management
INTRODUCTION
With the prevalence of obesity doubling since 1980 (1), there is an urgent need for effective, nutritionally balanced weight-loss strategies. Although many diets produce short-term weight loss, long-term dietary adherence and maintenance of weight loss are difficult to achieve (2). Approaches to reduce energy intake typically focus on limiting portions or food choices; however, such restrictive approaches may lead to hunger and dissatisfaction. In clinical trials, hunger has been associated with a lack of weight loss or with weight regain (3-5). Conversely, a dietary strategy that helps individuals control hunger by eating satisfying amounts of food could improve adherence and increase weight loss. One such approach is to encourage individuals to decrease the energy density of the diet by consuming low-energy-density foods.
The energy density (kcal/g) of the diet can be decreased by reducing intake of fat, which has a higher energy density (9 kcal/g) than does carbohydrate or protein (4 kcal/g). Incorporating water-rich foods such as fruit and vegetables into the diet can also reduce dietary energy density because water adds weight and volume to food without adding energy. For the same number of calories, individuals following a low-energy-density diet can eat a greater weight of food, and thus may experience less hunger than do individuals following a diet that restricts portions.
Multiple laboratory-based, controlled studies have been conducted in which participants were served test foods or diets that were varied in either fat content, the amount of fruit and vegetables, or both (6-13). The results of these studies indicated that these strategies to reduce dietary energy density were effective for decreasing energy intake for periods of up to 11 wk. Several clinical trials found that restricting fat consumption and combining fat reduction with increased fruit and vegetable intake both lead to weight loss (14-17). Although these trials did not assess the energy density of the diet (18), it is possible that the weight loss was related to a decrease in dietary energy density. The specific influence of dietary energy density on body weight, however, has not been extensively investigated. One trial that did assess both energy density and body weight found that instructing subjects to incorporate 2 servings of low-energy-density soup daily into a reduced-calorie diet led to greater weight loss than did incorporation of the same amount of energy as high-energy-density snack foods (19). These studies suggest that reducing the energy density of the diet is likely to be an effective strategy for weight management and should be explored further.
The present trial investigated the effects on weight loss of advice for reducing the energy density of the diet. Two different strategies were tested: one advised a reduction in fat intake and the other promoted an increased consumption of water-rich foods along with a reduction in fat intake. It was hypothesized that advising individuals to follow a reduced-fat diet would lead to a decrease in dietary energy density that would be associated with weight loss. In addition, it was hypothesized that promoting the incorporation of water-rich foods into a reduced-fat diet would lead to a further reduction in dietary energy density and energy intake, greater weight loss, and less hunger than would advising a reduced-fat diet alone.
SUBJECTS AND METHODS
Subjects
Potential subjects were recruited through flyers and newspaper advertisements. Eligibility was determined through a telephone interview, a physical screening, and questionnaires assessing symptoms of depression (20), symptoms of eating disorders (21-23), and the ability to safely engage in physical activity (24). Subjects were considered for inclusion in the study if they were women aged 20 to 60 y with a body mass index (BMI; in kg/m2) of 30 to 40; thus, all subjects were classified as obese (25). Respondents were excluded if they had blood pressure >140/90 mm Hg, serum triacylglycerols >400 mg/dL, or total cholesterol higher than the 90th percentile for their age (26); had a serious medical condition that precluded participation or any condition limiting physical activity; were pregnant or lactating; were taking a selective serotonin re-uptake inhibitor; had symptoms of depression or disordered eating; or were participating in a weight-loss program.
The sample size required in the trial was estimated by using data from obese women who participated in a weight-loss trial utilizing a dietary intervention with a similar frequency of contact (19). It was estimated that a sample of 35 subjects per intervention group would allow the detection of a 2.4-kg (5.3-lb) difference in weight loss between the groups by use of a repeated-measures analysis with a significance level of 0.05 and 80% power. On the basis of the previous trial, a subject attrition rate of 30% was predicted; thus, the aim was to enroll 50 subjects per intervention group.
One hundred ten women met the initial inclusion criteria and participated in a two-week run-in period (27, 28). During this period, 97 women met the secondary inclusion criteria (completing paperwork and attending sessions). These subjects were classified by age and severity of obesity (BMI) and were randomly assigned to 1 of 2 intervention groups through the use of a stratified permuted block design (Figure 1). Because this was to be the first intervention of this type to assess energy density, only women were tested to simplify the design and to enhance statistical power. Subjects provided written informed consent and were financially compensated for their participation. The protocol was approved by the Office for Research Protections of The Pennsylvania State University, and the clinical trial was conducted at the General Clinical Research Center at the University Park campus. This study was registered in the public trial registry maintained by the US National Library of Medicine (http://www.clinicaltrials.gov) as “Factors Affecting Caloric Regulation in Human Feeding” (NCT00108784).
FIGURE 1.
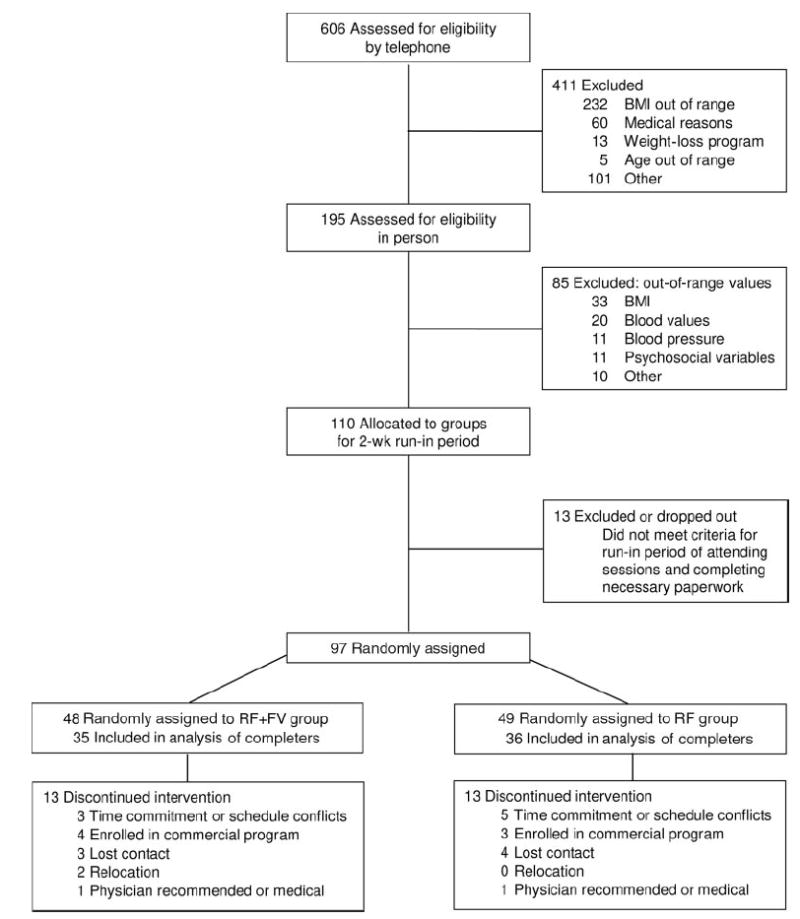
Flow diagram of subject enrollment, random assignment, and completion of the study protocol.
Study design
The year-long intervention was divided into 2 phases. During the first 6 mo (phase 1), participants met individually with a dietitian once per week, attending a maximum of 26 counseling sessions lasting ≈30 min. During the second 6 mo (phase 2), participants attended one small group session and one individual session with a dietitian each month. They attended a maximum of six 60-min small group sessions and six 15- to 30-min individual sessions during this phase.
Subjects in both intervention groups received the same amount of instruction on fat reduction, behavior change, physical activity, and the principles of their diets. To avoid counseling bias, individual dietitians spent a similar proportion of their time with subjects in each intervention group. Because the intervention required specific dietary changes, neither the participants nor the dietitians were blinded to intervention assignment. Although the participants were aware that there were 2 intervention groups, they were unaware of the dietary advice that was being provided to the other group.
Interventions
The goal for the subjects in both intervention groups was to reduce the energy density of their diets; however, the groups were taught different dietary strategies to achieve this goal. The reduced-fat (RF) group was advised to reduce fat intake, whereas the reduced-fat plus increased fruit and vegetable (RF+FV) group was advised to reduce fat intake and increase intake of water-rich foods, especially fruit and vegetables. Within each of the strategies, the subjects were taught to make food choices (reduced-fat foods or reduced-fat and water-rich foods) that were reduced in energy density and appropriate in portion size. Neither group was given daily limits for energy or fat intake; the subjects were instructed to eat ad libitum amounts of food while following the principles of their assigned diet.
Phase 1 (individual lessons)
During phase 1, the subjects received written materials and individual instruction from the dietitians. To achieve a reduced-fat diet, the subjects were instructed in cooking and recipe modifications and grocery shopping and dining-out strategies and were provided with meal and snack ideas. Subjects in the RF group were taught recommended serving sizes for common foods (29) and were advised to choose appropriate portion sizes. To match the 2 groups for the amount of instruction provided, the RF group was also counseled on nutrition topics important for women’s health (eg, iron intake).
Subjects in the RF+FV group were instructed in the same methods of dietary change as were the subjects in the RF group, but strategies to increase water-rich foods (eg, fruit, vegetables, and soups) were included in addition to strategies to reduce fat. These subjects were also taught about recommended serving sizes (29), but were encouraged to eat larger, satisfying portions of low-energy-density foods (fruit, vegetables, and soups) and recommended serving sizes of medium- and high-energy-density foods (30).
Subjects in both groups received the same behavior therapy recommendations based on social cognitive theory (31, 32). Behavioral therapy emphasized increasing self-efficacy for lifestyle changes. Some key topics incorporated into the intervention were self-monitoring, goal setting, social support networks, coping with emotional eating, managing stress and the environment, overcoming obstacles, problem solving, and handling setbacks.
Physical activity information for both groups focused on walking and using a pedometer to set goals and track progress. Subjects’ baseline step counts were determined over 7 days during the first week of the study. Subjects set goals to gradually increase their step counts by 20% of their baseline measure or 2000 steps per day (33, 34). The long-term goal for subjects was to reach 10 000 steps per day.
Phase 2 (group and individual lessons)
During phase 2, the subjects met in small groups within their intervention group (RF or RF+FV) to review the material presented during phase 1. The group lessons, which were led by the dietitians, comprised 6 topics for practical review: holiday eating, cooking and recipe modification, appropriate portion sizes, label reading, dining out, and grocery shopping. Although the primary focus of the group sessions was on the diet, physical activity was encouraged and all subjects received a handout at the conclusion of each session with practical information related to activity. During monthly individual sessions, the dietitians met with the subjects to review their diet records and discuss any questions or concerns.
Assessment of study outcomes
Changes in body weight and body composition
At each session, the subjects were weighed (within 0.1 kg) without shoes and while wearing light clothing on a calibrated scale. Standing height (within 0.5 cm) was measured at baseline and was confirmed at month 6. Body-composition measures were taken at baseline and months 3, 6, and 12. Percentage of body fat (within 0.1%) was measured by using bioelectrical impedance (Biodynamics model 310; Biodynamics Corporation, Seattle, WA) after the subjects had fasted for 12 h. The day before the impedance measurement, the subjects were instructed to abstain from alcohol and to drink plenty of fluids to be well hydrated (35). Waist circumference was measured (within 0.5 cm) by using the protocol of the third National Health and Nutrition Examination Survey (36, 37).
Diet composition
Before starting the study, the subjects attended a training session that taught them how to properly complete 3-d diet records; the session included special instructions on recording foods and beverages so that energy density could be correctly calculated. The subjects completed detailed diet records for 3 consecutive days (2 weekdays and 1 weekend day) every 2 wk during phase 1 and every 4 wk during phase 2. During the counseling sessions, the dietitians reviewed the diet records with the subjects to promote completeness and accuracy.
The diet records were analyzed by the Diet Assessment Center at The Pennsylvania State University, which has experience in calculating energy density from food records by using the Nutrition Data System for Research (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). In the analyses of total energy intake, all foods and caloric beverages were included. Fruit and vegetable intakes were analyzed both with and without those that were fried and dried to examine the effect of water-rich fruit and vegetables on weight loss.
Food energy density was calculated as the ratio between food energy (kcal) and food weight (g), excluding caloric beverages (such as milk and juices) as well as noncaloric beverages. The inclusion of beverages may disproportionately influence the calculation of energy density because beverages tend to be much lower in energy density than are most foods (38). Epidemiologic data indicate that including beverages may diminish associations between energy density and outcome variables because of increased within-person variance (38).
Hunger and satiety
On the same days that the subjects completed their diet records, they also rated their daily hunger and fullness 1.5 h after their evening meal (39-41) by using 100-mm visual analogue scales. For example, hunger was assessed with the question “How hungry did you feel today? ”; the scale was anchored on the left by “not at all hungry” and on the right by “extremely hungry.” Hunger and fullness were also rated immediately before and after each meal (breakfast, lunch, and dinner) on the first day that subjects completed diet records. Meal ratings were collected to assess the reliability of the single daily ratings.
Physical activity
Walking, the physical activity emphasized in the intervention, was measured by daily step counts. Subjects were provided with calibrated pedometers and were instructed in their use. They were required to record a minimum of 3 d of step counts every 2 wk in phase 1 and once per month in phase 2. The subjects recorded their step counts on the same 3 days that they recorded their food intake and rated their hunger and satiety.
Diet satisfaction
Subjects completed a Diet Satisfaction Questionnaire at baseline and months 3, 6, and 12 (42). The details of the development and validation of this instrument will be presented in a separate publication. Briefly, this questionnaire evaluates a variety of issues identified from the literature that may affect satisfaction with the diet. It assesses the following 7 factors, which were determined by principal components analyses: family dynamics, cost, preparation, convenience, healthy lifestyle, negative aspects, and preoccupation with food. The questionnaire provides a score for each of the factors as well as a global score for overall diet satisfaction. Items are coded so that a higher score indicates greater satisfaction or perceived benefit.
Psychosocial factors
The subjects completed several questionnaires at baseline, 6 mo, and 12 mo, including the Eating Inventory (43) to measure dietary restraint (the tendency to consciously restrict food intake to control body weight), disinhibition (the loss of control over eating in response to emotional or social cues), and hunger (the tendency to subjective feelings of hunger). At the same time points, the subjects also completed the Beck Depression Inventory II (20), which measures symptoms of depression in obese individuals, and the Eating Habits Checklist (23), which assesses the extent to which obese individuals experience binge eating problems.
Laboratory analyses and blood pressure
Blood samples were taken and analyzed for lipids and insulin at baseline and at 3, 6, and 12 mo and were analyzed for carotenoids at baseline, 6 mo, and 12 mo. The subjects were required to fast for 12 h and to abstain from alcohol for 48 h before providing a blood sample. Insulin and lipids were analyzed by Clinical Laboratory Services, Penn State Milton S Hershey Medical Center. Serum concentrations of total cholesterol and triacylglycerol were measured by enzymatic procedures (44). HDL cholesterol was measured after the precipitation of apolipoprotein B–containing lipoproteins in whole plasma by heparin-manganese chloride (45); LDL-cholesterol concentrations were calculated with use of the Friedewald equation (46). Carotenoids were analyzed by the University of California, San Diego, Cancer Center Nutrition Shared Resource. Plasma carotenoids were separated and quantified with an HPLC method (47). Blood pressure was measured twice per counseling session according to a standard protocol (48).
Statistical methods
All outcomes were analyzed by using a mixed model that included intervention group as a fixed effect. For the outcomes that were measured at many time points, including body weight, a random coefficients analysis was used to model the longitudinal response over time (49). Time was treated as a continuous covariate in the model, and lower-order polynomial factors of time were fitted if they were significantly related to the outcome. This model accounts for the correlation of the repeated measures within subjects by allowing the longitudinal response to vary randomly for each subject. Thus, the model can estimate changes in outcomes on the basis of all observations, including data for subjects who withdraw from the trial (50). By use of this method, the outcome of body weight was analyzed both for study completers and for all randomly assigned subjects. For outcomes such as dietary intakes and blood lipids, which were measured at few time points, the within-subject correlation was accounted for by using a covariance pattern in the mixed model. For all outcomes, the baseline value was included as a covariate; additionally, serum total cholesterol and BMI were included as covariates for the analysis of blood carotenoids (51).
Multivariate ANOVA was used to test macronutrient intakes as a percentage of total energy intake. Step-wise regression analyses were performed to predict weight loss on the basis of dietary measures (energy intake, energy density of food, total fat intake, combined fruit and vegetable intake, and total weight of food), physical activity (step counts), and scores from the Eating Inventory (dietary restraint, disinhibition, and hunger) at months 1, 2, 3, 6, 9, and 12. All analyses were performed by using SAS software (version 9; SAS Institute Inc, Cary, NC). Results were considered significant at P < 0.05; values are presented as means ± SEMs.
RESULTS
Subjects
There were no significant differences at baseline in characteristics of the subjects assigned to the 2 intervention groups (Table 1). Subjects ranged in age from 22 to 60 y, and 76% were employed full-time. At 12 mo, 71 subjects (73%) remained in the trial; these subjects are referred to as the study completers (Figure 1). The reasons given most frequently for withdrawal were inability to attend the counseling sessions because of schedule conflicts and relocation out of the area. There were no significant differences in baseline characteristics, including body weight, between the participants who completed the trial and those who withdrew.
TABLE 1.
Baseline characteristics in the 2 intervention groups of all randomly assigned subjects and for subjects who completed the trial1
| All randomly assigned subjects | Study completers | |||
|---|---|---|---|---|
| Characteristic | RF group
(n = 49) |
RF + FV group
(n = 48) |
RF group
(n = 36) |
RF + FV group
(n = 35) |
| Age (y) | 44.5 ± 1.32 | 45.3 ± 1.4 | 45.1 ± 1.5 | 46.7 ± 1.5 |
| Ethnicity [n (%)] | ||||
| White | 44 (90) | 47 (97) | 33 (92) | 34 (97) |
| Other | 5 (10) | 1 (3) | 3 (8) | 1 (3) |
| Educational level [n (%)] | ||||
| High school diploma or less | 14 (28) | 12 (25) | 10 (28) | 9 (26) |
| Some college to associate degree | 18 (37) | 15 (31) | 11 (30) | 10 (28) |
| Bachelor’s to graduate degree | 17 (35) | 21 (44) | 15 (42) | 16 (46) |
| Hunger score3 | 6.0 ± 0.4 | 6.1 ± 0.5 | 6.0 ± 0.5 | 6.1 ± 0.6 |
| Disinhibition score3 | 9.6 ± 0.5 | 10.0 ± 0.5 | 9.7 ± 0.6 | 10.2 ± 0.6 |
| Dietary restraint score3 | 9.4 ± 0.6 | 8.9 ± 0.5 | 9.5 ± 0.7 | 9.0 ± 0.6 |
| Depression score4 | 7.5 ± 0.9 | 6.8 ± 0.8 | 7.0 ± 1.0 | 6.9 ± 1.0 |
| Binge-eating score5 | 12.6 ± 0.9 | 13.3 ± 0.9 | 11.8 ± 1.0 | 13.1 ± 0.9 |
RF, reduced-fat intervention; RF + FV, reduced-fat plus increased fruit and vegetable intervention. Baseline values did not differ significantly between groups (unpaired t test), either for all randomly assigned subjects or for subjects who completed the trial.
¯x ± SEM (all such values).
Eating Inventory (40).
Beck Depression Inventory (18).
Eating Habits Checklist (21).
Changes in body weight and body composition
After 1 y, study completers in both intervention groups (n = 71) had lost a significant amount of weight compared with their baseline values (P < 0.0001; Table 2). Over the course of the trial, however, study completers who were advised to reduce their dietary fat intake and increase their intake of water-rich foods (RF+FV group) had a significantly different pattern of weight loss than did subjects who were advised to reduce their fat intake alone (RF group) (P = 0.002 for group × time interaction; Figure 2). During the first 6 mo of the trial (phase 1), completers in the RF+FV group lost more body weight (8.9 ± 0.8 kg, or 19.6 ± 1.8 lb) than did those in the RF group (6.7 ± 0.7 kg, or 14.7 ± 1.5 lb; P = 0.034), a difference of 33%. During the second 6 mo of the trial (phase 2), the pattern of weight change did not differ significantly between the groups (P = 0.056). In phase 2, when subject contact was less frequent, weight was well maintained; subjects in both groups regained an average of 0.7 ± 0.4 kg (1.5 ± 0.9 lb). At the end of the trial, mean weight loss was 7.9 ± 0.9 kg (17.4 ± 1.9 lb) in the RF+FV group and 6.4 ± 0.9 kg (14.1 ± 1.9 lb) in the RF group.
TABLE 2.
Body-composition measures in study completers over the course of 1 y1
| RF group
(n = 36) |
RF + FV group
(n = 35) |
Difference between groups | ||||
|---|---|---|---|---|---|---|
| Outcome at selected time points | Value | Change from
baseline value |
Value | Change from
baseline value |
Effect | P |
| Body weight (kg) | Group × time2 | 0.002 | ||||
| Baseline | 90.2 ± 1.4 3,4 | — | 90.8 ± 1.84 | — | ||
| 3 mo | 85.3 ± 1.4 | −4.9 ± 0.5 | 84.3 ± 1.7 | −6.5 ± 0.5 | ||
| 6 mo | 83.5 ± 1.5 | −6.7 ± 0.7 | 81.9 ± 1.7 | −8.9 ± 0.8 | ||
| 12 mo | 83.8 ± 1.7 | −6.4 ± 0.9 | 82.9 ± 2.0 | −7.9 ± 0.9 | ||
| BMI (kg/m2) | Group × time2 | <0.0001 | ||||
| Baseline | 33.3 ± 0.4 3,4 | — | 33.4 ± 0.54 | — | ||
| 3 mo | 31.4 ± 0.4 | −1.8 ± 0.2 | 31.0 ± 0.5 | −2.4 ± 0.2 | ||
| 6 mo | 30.8 ± 0.4 | −2.5 ± 0.3 | 30.2 ± 0.5 | −3.3 ± 0.3 | ||
| 12 mo | 30.9 ± 0.5 | −2.4 ± 0.4 | 30.5 ± 0.6 | −2.9 ± 0.4 | ||
| Body fat (%) | Group5 | 0.71 | ||||
| Time | <0.0001 | |||||
| Baseline | 39.2 ± 0.4 3,4 | — | 38.8 ± 0.54 | — | ||
| 3 mo | 37.6 ± 0.4 | −1.6 ± 0.3 | 37.0 ± 0.5 | −1.8 ± 0.3 | ||
| 6 mo | 36.5 ± 0.5 | −2.7 ± 0.4 | 35.6 ± 0.7 | −3.2 ± 0.5 | ||
| 12 mo | 36.6 ± 0.6 | −2.6 ± 0.5 | 36.2 ± 0.7 | −2.6 ± 0.5 | ||
| Body fat (kg) | Group5 | 0.44 | ||||
| Time | <0.0001 | |||||
| Baseline | 35.4 ± 0.7 3,4 | — | 35.4 ± 1.04 | — | ||
| 3 mo | 33.4 ± 0.7 | −1.9 ± 0.2 | 32.9 ± 1.0 | −2.6 ± 0.2 | ||
| 6 mo | 32.7 ± 0.7 | −2.7 ± 0.3 | 31.9 ± 1.0 | −3.5 ± 0.3 | ||
| 12 mo | 32.9 ± 0.8 | −2.5 ± 0.4 | 32.4 ± 1.1 | −3.1 ± 0.4 | ||
| Lean body mass (kg) | Group5 | 0.42 | ||||
| Time | 0.14 | |||||
| Baseline | 54.6 ± 0.7 3,4 | — | 55.0 ± 1.04 | — | ||
| 3 mo | 53.1 ± 0.8 | −1.4 ± 0.3 | 53.1 ± 0.8 | −1.9 ± 0.6 | ||
| 6 mo | 52.9 ± 0.9 | −1.6 ± 0.4 | 52.5 ± 0.8 | −2.5 ± 0.6 | ||
| 12 mo | 52.9 ± 0.9 | −1.6 ± 0.4 | 52.6 ± 0.9 | −2.4 ± 0.7 | ||
| Waist circumference (cm) | Group5 | 0.27 | ||||
| Time | 0.0002 | |||||
| Baseline | 106.5 ± 1.2 3,4 | — | 106.0 ± 1.84 | — | ||
| 3 mo | 102.8 ± 1.2 | −3.7 ± 0.8 | 102.1 ± 1.5 | −3.9 ± 1.1 | ||
| 6 mo | 104.1 ± 1.3 | −2.4 ± 1.2 | 101.6 ± 1.7 | −4.4 ± 1.3 | ||
| 12 mo | 99.7 ± 1.2 | −6.8 ± 1.1 | 97.8 ± 1.9 | −8.2 ± 1.3 | ||
All values are ¯x ± SEM. RF, reduced-fat intervention; RF + FV, reduced-fat plus increased fruit and vegetable intervention.
Random coefficients analysis was used to model the outcome across the 39 time points with control for baseline values. A significant group × time interaction indicates that the response over time differed between the groups. When all randomly assigned subjects (n = 97) were included in the analysis, the group × week interaction remained significant for body weight (P = 0.021) and BMI (P = 0.024).
Baseline values did not differ significantly between groups (unpaired t test).
Baseline values differed significantly from those at intervention time points according to a mixed model with repeated measures (P ≤ 0.0001). This indicates a within-group effect of time when the group × time interaction was significant, and a main effect of time when there was no significant interaction.
Differences in outcome between groups and over time were determined from a mixed linear model with repeated measures that included all available time points and that was controlled for baseline values. The group × time interaction was not significant.
FIGURE 2.
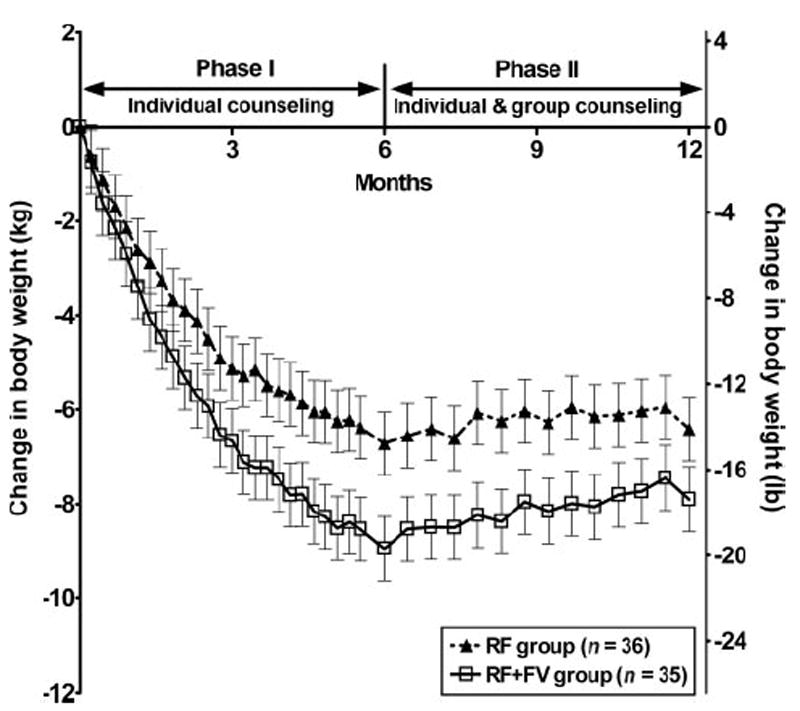
Mean (± SEM) change in body weight for study completers in the reduced-fat (RF) and reduced-fat plus increased fruit and vegetable (RF+FV) intervention groups over time. Random coefficients analysis was used to model the longitudinal response over time, with control for baseline values. The group × time interaction (P = 0.002) indicates that the response over time differed between the groups. The interaction remained significant (P = 0.021) when all randomly assigned subjects were included in the analysis. Baseline values did not differ significantly between the groups (unpaired t test).
The analysis of all randomly assigned subjects (n = 97) yielded conclusions similar to the analysis of study completers: over the course of the year, subjects in the RF+FV group lost significantly more body weight than did subjects in the RF group (P = 0.021). When all available data were included, the estimate of weight loss at the end of the trial was smaller in both groups (6.4 ± 0.8 kg in the RF+FV group and 4.9 ± 0.8 kg in the RF group). As in the analysis of completers, during phase 1 of the trial, there was a greater decline in body weight in the RF+FV group than in the RF group (P = 0.003), and during phase 2, the pattern of weight change did not differ significantly between the groups (P = 0.074).
Over the course of the study, study completers in both groups showed significant decreases in BMI, percentage body fat, and waist circumference from baseline values (P ≤ 0.0001; Table 2). Subjects in the RF+FV group had a significantly different pattern in the change in BMI than did subjects in the RF group (P < 0.0001 for group × time interaction). During phase 1, completers in the RF+FV group had a greater decrease in BMI than did those in the RF group (−3.3 ± 0.3 versus −2.5 ± 0.3; P = 0.0001), whereas during phase 2, changes in BMI did not differ significantly between the groups (−2.9 ± 0.4 in the RF+FV group and −2.4 ± 0.4 in the RF group; P = 0.514). After 1 y of intervention, 49% of subjects in the RF+FV group were no longer classified as obese (BMI ≥ 30) compared with 28% of subjects in the RF group (P = 0.071). Measured body fat decreased by an average of 7% for subjects in both groups. Apart from differences in weight loss and BMI, there were no significant differences between the groups in body-composition measures.
Reported diet composition, hunger and satiety, and physical activity
Examination of diet records indicated that study completers in both groups made dietary changes that resulted in a significant decrease in the energy density of their diets (P < 0.0001; Figure 3). Participants in the RF+FV group, however, reduced the energy density of their diets during the intervention to a greater extent (1.23 ± 0.02 kcal/g) than did those in the RF group (1.46 ± 0.02 kcal/g; P =0.019). Diet records showed that subjects in both groups significantly reduced their fat intake from 34.5 ± 0.6% of energy at baseline to 28.4 ± 0.4% during the intervention (P ≤ 0.0001). Fat intake did not differ significantly between the groups (P = 0.50; Table 3); this was expected because both groups received the same advice for dietary fat reduction. Similarly, reported intakes of carbohydrates and protein did not differ significantly between the groups during the intervention; the groups consumed a mean of 54.4 ± 0.5% of energy from carbohydrate and 18.0 ± 0.2% of energy from protein. The groups differed, however, in reported fruit and vegetable intake; this was expected because the dietary advice concerning fruit and vegetables differed between the groups. Subjects in the RF+FV group reported a greater intake of fruit and vegetables (P = 0.037; Table 3) than did those in the RF group; this was true regardless of whether fried and dried fruit and vegetables were included in the analysis. Subjects in the RF+FV group also consumed significantly more dietary fiber (g/1000 kcal; P = 0.001).
FIGURE 3.
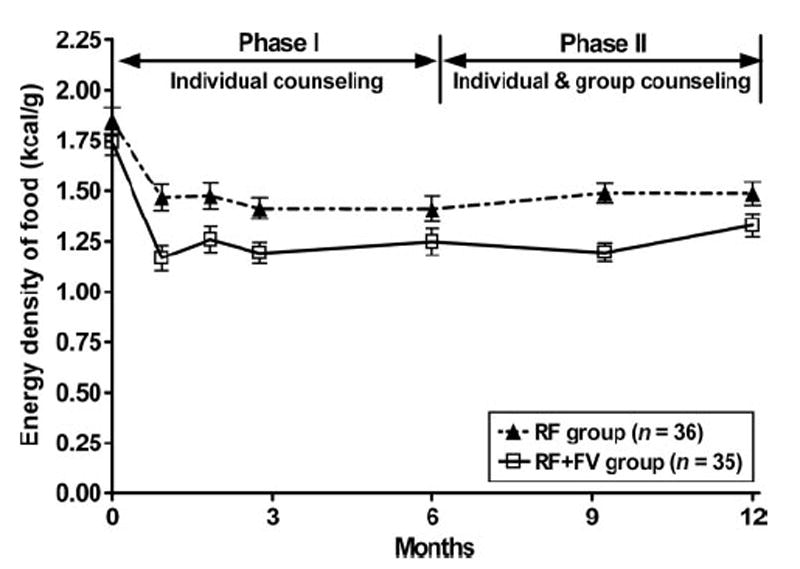
Mean (± SEM) food energy density (kcal/g) for study completers in the reduced-fat (RF) and reduced-fat plus increased fruit and vegetable (RF+FV) intervention groups over time. Differences in dietary energy density between the groups were determined from a mixed linear model with repeated measures, by using all available time points and with control for baseline values. The group × time interaction was significant (P = 0.019), which indicated that dietary energy density was significantly less in the RF+FV group than in the RF group during the intervention. Baseline values did not differ significantly between the groups (unpaired t test).
TABLE 3.
Dietary measures in study completers over the course of 1 y1
| RF group
(n = 36) |
RF + FV group
(n = 35) |
Difference between groups | ||||
|---|---|---|---|---|---|---|
| Outcome | Value | Change from
baseline value |
Value | Change from
baseline value |
Effect | P |
| Energy intake of food and beverages (kcal/d) | Group2 | 0.71 | ||||
| Time | <0.0001 | |||||
| Baseline | 1836 ± 683,4 | — | 1937 ± 784 | — | ||
| 1 mo | 1430 ± 60 | −406 ± 84 | 1376 ± 47 | −562 ± 76 | ||
| 2 mo | 1384 ± 62 | −452 ± 87 | 1336 ± 64 | −601 ± 85 | ||
| 3 mo | 1374 ± 62 | −462 ± 79 | 1358 ± 60 | −580 ± 81 | ||
| 6 mo | 1266 ± 62 | −570 ± 79 | 1440 ± 60 | −498 ± 81 | ||
| 9 mo | 1351 ± 71 | −486 ± 89 | 1388 ± 76 | −549 ± 98 | ||
| 12 mo | 1307 ± 62 | −525 ± 80 | 1437 ± 60 | −501 ± 81 | ||
| Food intake (g/d) | Group × time2 | 0.025 | ||||
| Baseline | 975 ± 473 | — | 1068 ± 49 | — | ||
| 1 mo | 936 ± 435 | −39 ± 44 | 1192 ± 68 | 124.6 ± 52 | ||
| 2 mo | 894 ± 425 | −81 ± 38 | 1101 ± 72 | 33.3 ± 62 | ||
| 3 mo | 936 ± 47 | −39 ± 48 | 1117 ± 69 | 49.0 ± 54 | ||
| 6 mo | 892 ± 515 | −83 ± 40 | 1136 ± 63 | 68.2 ± 54 | ||
| 9 mo | 869 ± 495 | −108 ± 50 | 1132 ± 63 | 64.6 ± 54 | ||
| 12 mo | 873 ± 515 | −85 ± 41 | 1073 ± 58 | 5.3 ± 53 | ||
| Energy density of food only (kcal/g) | Group × time2 | 0.019 | ||||
| Baseline | 1.85 ± 0.073,4 | — | 1.74 ± 0.064 | — | ||
| 1 mo | 1.47 ± 0.065 | −0.38 ± 0.09 | 1.17 ± 0.06 | −0.57 ± 0.06 | ||
| 2 mo | 1.47 ± 0.065 | −0.37 ± 0.09 | 1.26 ± 0.07 | −0.48 ± 0.07 | ||
| 3 mo | 1.41 ± 0.055 | −0.43 ± 0.08 | 1.19 ± 0.05 | −0.55 ± 0.05 | ||
| 6 mo | 1.41 ± 0.06 | −0.44 ± 0.07 | 1.25 ± 0.07 | −0.49 ± 0.07 | ||
| 9 mo | 1.49 ± 0.055 | −0.35 ± 0.07 | 1.20 ± 0.04 | −0.54 ± 0.06 | ||
| 12 mo | 1.49 ± 0.07 | −0.36 ± 0.08 | 1.33 ± 0.04 | −0.41 ± 0.06 | ||
| Fat intake (g/d) | Group2 | 0.50 | ||||
| Time | <0.0001 | |||||
| Baseline | 68.9 ± 3.33,4 | — | 77.4 ± 4.14 | — | ||
| 1 mo | 46.3 ± 3.4 | −22.6 ± 5.2 | 42.6 ± 2.7 | −34.8 ± 4.3 | ||
| 2 mo | 43.8 ± 3.1 | −25.1 ± 4.7 | 43.5 ± 3.1 | −33.9 ± 4.4 | ||
| 3 mo | 41.5 ± 3.2 | −27.5 ± 5.0 | 42.9 ± 2.6 | −34.5 ± 4.5 | ||
| 6 mo | 39.4 ± 2.2 | −29.5 ± 3.5 | 48.8 ± 3.7 | −28.6 ± 4.4 | ||
| 9 mo | 42.7 ± 2.8 | −26.2 ± 4.1 | 45.6 ± 3.0 | −31.8 ± 4.8 | ||
| 12 mo | 43.7 ± 2.8 | −25.5 ± 3.9 | 47.9 ± 2.8 | −29.5 ± 5.1 | ||
| Fruit and vegetable intake (g/d) | Group × time2 | 0.037 | ||||
| Baseline | 277.1 ± 22.03 | — | 364.7 ± 27.6 | — | ||
| 1 mo | 345.6 ± 23.75,6 | 68.5 ± 22.4 | 502.8 ± 41.76 | 138.2 ± 38.5 | ||
| 2 mo | 315.5 ± 26.25,6 | 38.4 ± 27.2 | 464.0 ± 49.16 | 99.3 ± 44.8 | ||
| 3 mo | 327.3 ± 27.85,6 | 50.2 ± 28.6 | 457.0 ± 34.86 | 92.4 ± 34.6 | ||
| 6 mo | 286.3 ± 23.15 | 9.2 ± 26.7 | 454.8 ± 40.4 | 90.2 ± 36.1 | ||
| 9 mo | 273.9 ± 22.85 | −3.2 ± 22.2 | 446.2 ± 33.8 | 81.5 ± 30.4 | ||
| 12 mo | 302.3 ± 25.5 | 26.8 ± 24.1 | 416.9 ± 29.3 | 52.3 ± 32.1 | ||
| Dietary fiber intake (g/1000 kcal) | Group × time2 | 0.001 | ||||
| Baseline | 8.4 ± 0.63,4 | — | 9.0 ± 0.54 | — | ||
| 1 mo | 10.4 ± 0.55 | 2.0 ± 0.7 | 13.3 ± 0.8 | 4.3 ± 0.6 | ||
| 2 mo | 10.5 ± 0.55 | 2.0 ± 0.7 | 13.3 ± 0.8 | 4.3 ± 0.8 | ||
| 3 mo | 10.4 ± 0.5 | 2.0 ± 0.6 | 12.0 ± 0.6 | 3.0 ± 0.7 | ||
| 6 mo | 10.9 ± 0.5 | 2.5 ± 0.6 | 11.7 ± 0.7 | 2.7 ± 0.7 | ||
| 9 mo | 10.7 ± 0.6 | 2.2 ± 0.6 | 12.1 ± 0.5 | 3.1 ± 0.5 | ||
| 12 mo | 10.9 ± 0.8 | 2.4 ± 0.6 | 12.0 ± 0.7 | 3.1 ± 0.7 | ||
| Physical activity (steps/d) | Group7 | 0.50 | ||||
| Time | <0.0001 | |||||
| Baseline | 5984 ± 5033,4 | — | 6731 ± 5854 | — | ||
| 1 mo | 7855 ± 503 | 1871 ± 584 | 8788 ± 413 | 2057 ± 532 | ||
| 2 mo | 8913 ± 382 | 2929 ± 526 | 8937 ± 490 | 2206 ± 681 | ||
| 3 mo | 8845 ± 398 | 2861 ± 553 | 9253 ± 528 | 2522 ± 659 | ||
| 6 mo | 8715 ± 427 | 2731 ± 609 | 8812 ± 504 | 2081 ± 720 | ||
| 9 mo | 8467 ± 498 | 2483 ± 604 | 8348 ± 490 | 1726 ± 698 | ||
| 12 mo | 9210 ± 426 | 3226 ± 573 | 9556 ± 453 | 2758 ± 635 | ||
All values are ¯x ± SEM. RF, reduced-fat intervention; RF + FV, reduced-fat plus increased fruit and vegetable intervention.
Differences in outcome between groups and over time were determined from a mixed linear model with repeated measures that included all available time points and that was controlled for baseline values. Unless indicated, the group × time interaction was not significant.
Baseline values did not differ significantly between groups (unpaired t test).
Baseline values differed significantly from those at all intervention time points (P ≤ 0.0001), as indicated by the main effect of time in a mixed model with repeated measures.
Time points at which values differed significantly between the groups with a significant group × time interaction.
Values at indicated time points differed significantly from those at baseline (P ≤ 0.0005), as indicated by the main effect of time in a mixed model with repeated measures.
Random coefficients analysis was used to model the outcome across the 39 time points with control for baseline values. The group × time interaction was not significant.
Diet records during the intervention showed a reduction in energy intake from baseline values of ≈500 kcal/d (P <0.0001; Table 3), which did not differ significantly between the groups. The difference in weight loss between the groups corresponded with an estimated difference in energy intake of ≈100 kcal/d, whereas the SE of the difference in energy intake between the groups was 113 kcal. Thus, given the variability in reported energy intake, we were not able to detect a difference in energy intake between the groups that would account for the difference in weight loss.
There was a significant difference between the groups in the weight of food consumed during the trial. Subjects in the RF+FV group reported consuming significantly more food than did subjects in the RF group (P = 0.025; Table 3). Specifically, the subjects who were encouraged to eat satisfying portions of low-energy-density, water-rich foods (RF+FV group) consumed a mean of 225 g (25%) more food daily.
Daily ratings of hunger, as measured by visual analogue scales at the end of the day, did not differ significantly from baseline for subjects in the RF group. Over the course of the study, however, hunger ratings reported by subjects in the RF+FV group were significantly lower than their ratings at baseline (P = 0.030). This resulted in significantly different ratings for hunger between the groups during the intervention (RF+FV group: 46.7 ± 2.2; RF group: 53.5 ± 2.2; P = 0.003). Ratings for fullness did not differ significantly between the groups. Analyses to assess the reliability of the daily measures showed that daily hunger ratings were significantly correlated with hunger ratings collected before and after meals; these results will be reported in a separate publication.
Physical activity, as measured by step counts, increased significantly from baseline for subjects in both groups (P < 0.0001; Table 3). Step counts did not differ significantly between the groups during the trial (P = 0.50); this was expected because the advice given for physical activity was the same for both groups. Subjects in both groups increased their daily step counts by an additional 2427 ± 100 steps/d to a total of 8735 ± 70 steps/d over the course of the trial. This exceeded the goal of an additional 2000 steps/d and increased the average daily step count by 38% from baseline, an increase that was maintained over the course of the year.
Questionnaires
Overall scores for satisfaction were significantly higher for the intervention diets (3.6 ± 0.1) than for the baseline diets (3.2 ± 0.1) for subjects in both groups (maximum score 5.0; P < 0.0001). There were no significant differences between the 2 dietary approaches in any of the ratings of diet satisfaction. Subjects in both groups rated their intervention diets as providing greater health benefits, having fewer negative aspects, working better within the family context, and leading to less preoccupation with food than did their baseline diets (P < 0.0001). Ratings of perceived cost effectiveness, preparation time, and convenience were lower for both diet strategies than for the diets consumed at baseline (all P < 0.05), but there were no significant differences between the groups.
Although there were significant changes from baseline on scores from the Eating Inventory, the Beck Depression Inventory II, and the Eating Habits Checklist, there were no significant differences between the groups. Analysis of the Eating Inventory showed that for all subjects there was a significant increase in dietary restraint scores at 6 and 12 mo (mean score 14.7 ± 0.3; P < 0.0001), whereas scores for disinhibition and hunger decreased at these time points (mean scores: 7.3 ± 0.3 and 4.4 ± 0.3, respectively; P < 0.0001). There were significant improvements in depression scores for subjects in both groups (mean score: 4.6 ± 0.4; P < 0.0001), as well as a significant decrease in scores for degree of binge-eating problems at both 6 and 12 mo (mean score: 7.7 ± 0.4; P < 0.0001).
Laboratory outcomes and blood pressure
Subjects showed beneficial changes from baseline in several physiologic values, even though baseline values were within normal ranges. Significant changes from baseline were seen in both groups for insulin, and at some time points for all cholesterol measures except LDL cholesterol (Table 4). Improvements in physiologic measures were significantly greater in the RF+FV group than in the RF group at various time points during the study for insulin, non-HDL cholesterol, and triacylglycerols (all P < 0.044; Table 4). For LDL cholesterol and HDL cholesterol, no significant differences were found between the groups.
TABLE 4.
Insulin, lipid, and blood pressure measures in study completers over the course of 1 y1
| RF group
(n = 36) |
RF + FV group
(n = 35) |
Difference between groups | ||||
|---|---|---|---|---|---|---|
| Outcome | Value | Change from
baseline value |
Value | Change from
baseline value |
Effect | P |
| Insulin (μU/mL) | Group × time2 | 0.016 | ||||
| Baseline | 20.3 ± 1.03 | 20.9 ± 1.2 | ||||
| 3 mo | 17.6 ± 1.04,5 | −2.7 ± 0.8 | 15.9 ± 0.84 | −5.1 ± 0.8 | ||
| 6 mo | 17.8 ± 0.94 | −2.6 ± 0.9 | 18.6 ± 1.24 | −2.4 ± 0.9 | ||
| 12 mo | 17.7 ± 1.34 | −2.8 ± 1.1 | 17.4 ± 1.04 | −3.6 ± 1.1 | ||
| Total cholesterol (mg/dL) | Group2 | 0.055 | ||||
| Time | 0.024 | |||||
| Baseline | 201.7 ± 5.63 | 197.0 ± 5.1 | ||||
| 3 mo | 203.1 ± 6.8 | +3.3 ± 4.2 | 187.2 ± 6.0 | −9.8 ± 4.2 | ||
| 6 mo | 196.2 ± 6.14 | −4.1 ± 3.8 | 187.0 ± 5.54 | −10.0 ± 3.8 | ||
| 12 mo | 206.9 ± 6.8 | +5.5 ± 4.6 | 194.2 ± 6.9 | −3.8 ± 4.7 | ||
| LDL cholesterol (mg/dL) | Group2 | 0.12 | ||||
| Time | 0.16 | |||||
| Baseline | 119.7 ± 4.43 | 122.1 ± 4.4 | ||||
| 3 mo | 117.0 ± 4.8 | −0.2 ± 4.0 | 116.5 ± 5.2 | −5.6 ± 3.9 | ||
| 6 mo | 117.2 ± 5.3 | −0.7 ± 3.4 | 116.1 ± 4.6 | −6.0 ± 3.3 | ||
| 12 mo | 127.7 ± 5.7 | +7.4 ± 4.0 | 119.2 ± 5.4 | −4.2 ± 3.9 | ||
| HDL cholesterol (mg/dL) | Group2 | 0.09 | ||||
| Time | 0.004 | |||||
| Baseline | 52.7 ± 2.63 | 47.1 ± 1.7 | ||||
| 3 mo | 49.5 ± 2.24 | −3.3 ± 1.4 | 46.0 ± 1.84 | −1.1 ± 0.7 | ||
| 6 mo | 52.6 ± 2.3 | −0.3 ± 1.3 | 48.8 ± 1.7 | +1.7 ± 0.8 | ||
| 12 mo | 54.1 ± 2.84 | +1.3 ± 1.3 | 51.1 ± 2.24 | +3.6 ± 0.9 | ||
| Non-HDL cholesterol (mg/dL) | Group × time2 | 0.043 | ||||
| Baseline | 149.0 ± 5.23 | 149.9 ± 5.5 | ||||
| 3 mo | 153.6 ± 6.75 | 6.6 ± 4.0 | 141.2 ± 5.9 | −8.7 ± 4.8 | ||
| 6 mo | 143.6 ± 5.94 | −3.7 ± 3.6 | 138.2 ± 5.54 | −11.7 ± 3.8 | ||
| 12 mo | 152.8 ± 6.25 | 3.8 ± 5.0 | 143.1 ± 6.9 | −7.8 ± 4.3 | ||
| HDL:total cholesterol | Group2 | 0.044 | ||||
| Time | 0.022 | |||||
| Baseline | 4.1 ± 0.23 | 4.4 ± 0.2 | ||||
| 3 mo | 4.4 ± 0.2 | +0.4 ± 0.1 | 4.2 ± 0.2 | −0.1 ± 0.2 | ||
| 6 mo | 3.9 ± 0.24 | −0.1 ± 0.1 | 4.0 ± 0.24 | −0.4 ± 0.1 | ||
| 12 mo | 4.1 ± 0.2 | −0.01 ± 0.1 | 4.0 ± 0.2 | −0.4 ± 0.1 | ||
| Triacylglycerols (mg/dL) | Group × time2 | 0.017 | ||||
| Baseline | 149.1 ± 13.43 | 139.1 ± 11.2 | ||||
| 3 mo | 184.0 ± 22.34,5 | +38.3 ± 12.1 | 123.9 ± 7.9 | −15.1 ± 12.1 | ||
| 6 mo | 140.6 ± 15.95 | −6.1 ± 9.5 | 111.2 ± 7.94 | −27.9 ± 9.5 | ||
| 12 mo | 135.8 ± 14.0 | −13.3 ± 11.7 | 119.6 ± 12.0 | −17.0 ± 11.8 | ||
| Systolic blood pressure (mm Hg) | Group6 | 0.85 | ||||
| Time | 0.004 | |||||
| Baseline | 119.9 ± 1.73 | 120.2 ± 1.7 | ||||
| 3 mo | 108.6 ± 1.94 | −11.3 ± 1.6 | 110.1 ± 2.04 | −10.1 ± 1.3 | ||
| 6 mo | 112.5 ± 2.04 | −7.4 ± 1.7 | 113.6 ± 2.04 | −6.4 ± 1.6 | ||
| 12 mo | 112.8 ± 2.04 | −7.0 ± 1.8 | 113.6 ± 2.14 | −6.6 ± 1.4 | ||
| Diastolic blood pressure (mm Hg) | Group6 | 0.96 | ||||
| Time | <0.0001 | |||||
| Baseline | 78.4 ± 1.13 | 79.3 ± 1.0 | ||||
| 3 mo | 71.5 ± 1.14 | −6.9 ± 1.1 | 72.3 ± 1.24 | −7.0 ± 1.1 | ||
| 6 mo | 72.4 ± 1.54 | −5.9 ± 1.3 | 73.5 ± 1.24 | −5.6 ± 1.0 | ||
| 12 mo | 70.3 ± 1.54 | −8.0 ± 1.3 | 72.7 ± 1.44 | −6.6 ± 1.3 | ||
All values are ¯x ± SEM. RF, reduced-fat intervention; RF + FV, reduced-fat plus increased fruit and vegetable intervention.
Differences in outcome between groups and over time were determined from a mixed linear model with repeated measures that included all available time points and that was controlled for baseline values. Unless indicated, the group × time interaction was not significant.
Baseline values did not differ significantly between groups (unpaired t test).
Values at indicated time points differed significantly from those at baseline (P ≤ 0.001), as indicated by the main effect of time in a mixed model with repeated measures.
Time points at which values differed significantly between the groups with a significant group × time interaction.
Random coefficients analysis was used to model the outcome across the 39 time points with control for baseline values. The group × time interaction was not significant.
There were increases in both groups over 1 y in serum α-carotene, lycopene, and β-cryptoxanthin concentrations (all P < 0.03). The RF+FV group showed a significantly greater increase during the trial in α-carotene than did the RF group (54% increase versus 37% increase; P = 0.023). For lutein and zeaxanthin concentrations, there was a significant change between groups over time; subjects in the RF+FV group had greater concentrations of lutein and zeaxanthin than did subjects in the RF group at 6 mo only (P = 0.047).
For all subjects, there was a significant decrease from baseline in systolic and diastolic blood pressure (Table 4; P < 0.001); however, there were no significant differences between the groups in the blood pressure measures over time (both P > 0.85).
Factors predicting weight loss
To investigate which factors were most predictive of weight loss, stepwise regression analyses were performed by using data from dietary measures (energy intake, energy density of food, total fat intake, combined fruit and vegetable intake, and total weight of food), physical activity, and scores from the Eating Inventory. The primary predictor of weight loss at month 1 was the energy density of food (R2 = 0.16) along with step counts (R2 = 0.05; both P < 0.040). Together, these factors accounted for 21% of the variability in weight loss. Energy density was the only significant predictor of weight loss at month 2 (R2 = 0.07; P = 0.022) and also at month 3 (R2 = 0.17; P = 0.0004). The strength of the relation (regression coefficient) between food energy density and weight loss increased significantly from month 1 to month 3 (P = 0.025).
At months 6, 9, and 12, fruit and vegetable intake became the primary predictor of weight loss from baseline (R2 = 0.26, 0.16, and 0.20, respectively; all P < 0.0006). The strength of the relation (regression coefficient) between fruit and vegetable intake and weight loss did not vary significantly from month 6 to month 12 (P = 0.92). Secondarily, the increase in the Eating Inventory dietary restraint score and the decrease in the Eating Inventory hunger score predicted weight loss during the second phase of the study (all P < 0.029). These scores predicted an additional 20% of the variability in weight loss at month 6 and an additional 11% at month 12.
DISCUSSION
In this randomized controlled trial with obese women, advice to reduce dietary energy density led to significant weight loss, most of which was maintained over 1 y. Participants in both intervention groups lost weight without being given specific limits for daily intakes of energy or fat, and weight loss led to beneficial changes in serum lipids and plasma insulin. The magnitude of weight lost was significantly affected by the dietary strategy that the participants were instructed to follow. Participants who were advised to decrease the energy density of their diets by adding water-rich foods and reducing their fat intake lost 33% more weight at 6 mo than did those who were advised to decrease their fat intake alone. The 2 strategies were similar in effectiveness for maintaining weight during the second 6 mo of the study. Thus, advice to reduce the energy density of the diet, especially by combining fat reduction with increased consumption of fruit and vegetables, was effective for weight loss and maintenance.
Analysis of diet records indicated that both strategies resulted in a decrease in dietary energy density. By following the same dietary advice for fat reduction, participants in both groups decreased their reported consumption of fat from 34% to 28% of energy. Participants who received additional advice to consume more water-rich foods increased their reported fruit and vegetable intake, which contributed to a further reduction in energy density. Over the course of the intervention, subjects in this group also reported eating 25% more food by weight and rated hunger significantly lower than did subjects who were advised only to reduce their fat intake. It is probable that greater food intake and reduced hunger led to better adherence to the diet, thus contributing to the greater weight loss.
Previous clinical trials found that advice to reduce dietary fat was associated with decreased energy intake and body weight (52, 53). The results of the present study provide further evidence of the effectiveness of fat reduction for weight loss. In previous trials of dietary fat reduction, however, dietary energy density was not assessed. It is important to evaluate the impact of fat reduction on energy density because these 2 dietary factors can change independently. Several shorter-term studies indicated that the effect of fat reduction on energy intake is mediated by a reduction in energy density (54). The present trial further suggests that fat reduction leads to weight loss by contributing to a reduction in the energy density of the diet. Regression analysis showed that dietary energy density was the main predictor of weight loss during the period of greatest weight change, and that dietary fat intake did not independently predict weight loss after energy density was accounted for. It is critical that future studies of the effects of macronutrients on body weight evaluate the contribution of energy density to the outcome.
Although fat reduction is one strategy for reducing dietary energy density, the addition of water-rich foods can further reduce energy density. In the present trial, advice to incorporate water-rich foods into a reduced-fat diet was more effective in controlling hunger, reducing body weight, and improving some physiologic measures than was simply following a reduced-fat diet. In addition to these outcomes, there are several other advantages of advice to increase consumption of water-rich foods. Such advice presents a positive message that balances the restrictive message to consume less fat; it has been shown that using positive messages results in greater dietary changes (55). Increasing the consumption of fruit and vegetables is also consistent with current dietary guidelines and is associated with high diet quality (56). Promoting increased fruit and vegetable intake for weight management may lead to beneficial effects on other health-related outcomes, such as reduction of risk for chronic illness (57-60).
Although there are many advantages to fruit and vegetable consumption, factors such as cost, convenience, and preparation time are often cited as barriers to increased intake (61). In this trial, although subjects in the 2 groups differed in reported consumption of fruit and vegetables, they gave similar ratings of satisfaction with the 2 dietary approaches, particularly for cost, convenience, and food preparation. These data indicate that specific instruction in techniques for increasing fruit and vegetable intake was both effective and well accepted by the participants.
In addition to the strategies to reduce dietary energy density, the effectiveness of the weight-loss interventions was enhanced by the physical activity component. Participants in both groups exceeded their goal of an additional 2000 steps/d and increased their daily step counts by an average of 38%. Subjects maintained a significant increase in step counts over the course of the intervention, which confirms continued adherence to the program for 1 y. These sustained behavioral changes are likely to have contributed to the maintenance of weight loss (62-64).
The detailed diet records kept by the participants showed that energy intake during the intervention declined significantly from baseline values in both groups. Between the groups, however, there were no significant differences in reported energy intake that corresponded with the differences in weight loss. This may be explained by the large variability in daily energy intake, which limited the statistical power to detect modest differences in intake given the number of subjects in the trial. Conversely, the much larger differences between groups in the weight of food consumed allowed for the detection of significant changes in both food weight and dietary energy density. In self-reported diet records from obese subjects there are also issues regarding underreporting of intake (65) and compliance bias (66, 67). Because all participants in this trial were obese and received the same amount of dietary information and counseling, the potential for underreporting and bias was likely to have been similar in both groups. The advice given to one group to increase intake of water-rich foods may have influenced those participants to report a greater intake than they were actually consuming. It is unlikely that compliance bias fully explains the reported increase in fruit and vegetable consumption in this group, however, because the increase corresponded with an increase in plasma carotenoids.
The promising results of this trial indicate the need to investigate the effects of low-energy-density diets in a larger multicenter trial, which could include a diversity of populations and a variety of settings. Future studies should also investigate the effectiveness of a less intensive intervention. Adherence to both the diet and physical activity advice may have been improved by the frequent contact with dietitians, which has been reported to increase compliance in other studies (68, 69). Of additional interest is determining whether adherence to a diet low in energy density persists beyond 1 y. Clinical data from a weight-loss program in which individuals were advised to add low-energy-density foods to a reduced-fat, energy-restricted diet showed that weight loss was well maintained for 2 to 6 y after treatment (70, 71), and at 2 y maintenance was associated with a low-energy-density dietary pattern (72).
The 2 strategies for reducing energy density that were tested in this trial were both effective in reducing body weight and maintaining weight loss without prescribing limits for energy or fat intake. Diets that are low in energy density, particularly those that encourage increased consumption of water-rich foods, allow individuals to eat adequate amounts of food while restricting energy intake, which helps to control hunger. Because a wide variety of foods can be included in a diet that is reduced in energy density (56, 73), this type of eating plan can be adapted to individual food preferences. Individuals should be encouraged to find a low-energy-density eating pattern that is nutritionally balanced and that can be sustained for lifelong weight management.
Acknowledgments
We are grateful to Kitti Halverson and Denise Shaffer Taylor for their assistance with the development of the nutrition education materials and their dedication to working with the study participants; the staff of the General Clinical Research Center; the staff of the Diet Assessment Center at Penn State University for the analysis of the diet records; and Diane C Mitchell and Cheryl L Rock for assistance with carotenoid analyses. We especially thank our study participants for their commitment to the study.
Footnotes
Supported by National Institutes of Health grants R37DK039177 and M01RR10732.
None of the authors had any conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Makris AP, Foster GD. Dietary approaches to the treatment of obesity. Psychiatr Clin North Am. 2005;28:117–39. viii–ix. doi: 10.1016/j.psc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Pasman WJ, Saris WH, Westerterp-Plantenga MS. Predictors of weight maintenance. Obes Res. 1999;7:43–50. doi: 10.1002/j.1550-8528.1999.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 5.Cuntz U, Leibbrand R, Ehrig C, Shaw R, Fichter MM. Predictors of post-treatment weight reduction after in-patient behavioral therapy. Int J Obes Relat Metab Disord. 2001;25(suppl):S99–101. doi: 10.1038/sj.ijo.0801710. [DOI] [PubMed] [Google Scholar]
- 6.Rolls BJ, Bell EA, Castellanos VH, Chow M, Pelkman CL, Thorwart ML. Energy density but not fat content of foods affected energy intake in lean and obese women. Am J Clin Nutr. 1999;69:863–71. doi: 10.1093/ajcn/69.5.863. [DOI] [PubMed] [Google Scholar]
- 7.Duncan KH, Bacon JA, Weinsier RL. The effects of high and low energy density diets on satiety, energy intake, and eating time of obese and nonobese subjects. Am J Clin Nutr. 1983;37:763–7. doi: 10.1093/ajcn/37.5.763. [DOI] [PubMed] [Google Scholar]
- 8.Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr. 1998;67:412–20. doi: 10.1093/ajcn/67.3.412. [DOI] [PubMed] [Google Scholar]
- 9.Rolls BJ, Bell EA. Dietary approaches to the treatment of obesity. Med Clin North Am. 2000;84:401–18. doi: 10.1016/s0025-7125(05)70228-5. [DOI] [PubMed] [Google Scholar]
- 10.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr. 2001;73:1010–8. doi: 10.1093/ajcn/73.6.1010. [DOI] [PubMed] [Google Scholar]
- 11.Kendall A, Levitsky DA, Strupp BJ, Lissner L. Weight loss on a low-fat diet: consequence of the imprecision of the control of food intake in humans. Am J Clin Nutr. 1991;53:1124–9. doi: 10.1093/ajcn/53.5.1124. [DOI] [PubMed] [Google Scholar]
- 12.Lissner L, Levitsky DA, Strupp BJ, Kalkwarf HJ, Roe DA. Dietary fat and the regulation of energy intake in human subjects. Am J Clin Nutr. 1987;46:886–92. doi: 10.1093/ajcn/46.6.886. [DOI] [PubMed] [Google Scholar]
- 13.Stubbs RJ, Johnstone AM, O’Reilly LM, Barton K, Reid C. The effect of covertly manipulating the energy density of mixed diets on ad libitum food intake in ‘pseudo free-living’ humans. Int J Obes. 1998;22:980–7. doi: 10.1038/sj.ijo.0800715. [DOI] [PubMed] [Google Scholar]
- 14.Henderson MM, Kushi LH, Thompson DJ, et al. Feasibility of a randomized trial of a low-fat diet for the prevention of breast cancer: dietary compliance in the Women’s Health Trial Vanguard Study. Prev Med. 1990;19:115–33. doi: 10.1016/0091-7435(90)90014-b. [DOI] [PubMed] [Google Scholar]
- 15.Baer JT. Improved plasma cholesterol levels in men after a nutrition education program at the worksite. J Am Diet Assoc. 1993;93:658–63. doi: 10.1016/0002-8223(93)91672-d. [DOI] [PubMed] [Google Scholar]
- 16.Lanza E, Schatzkin A, Daston C, et al. Implementation of a 4-y, high-fiber, high-fruit-and-vegetable, low-fat dietary intervention: results of dietary changes in the Polyp Prevention Trial. Am J Clin Nutr. 2001;74:387–401. doi: 10.1093/ajcn/74.3.387. [DOI] [PubMed] [Google Scholar]
- 17.Gambera PJ, Schneeman BO, Davis PA. Use of the Food Guide Pyramid and US Dietary Guidelines to improve dietary intake and reduce cardiovascular risk in active-duty Air Force members. J Am Diet Assoc. 1995;95:1268–73. doi: 10.1016/s0002-8223(95)00334-7. [DOI] [PubMed] [Google Scholar]
- 18.Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004;62:1–17. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res. 2005;13:1052–60. doi: 10.1038/oby.2005.123. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 21.Garner DM, Garfinkel PE. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med. 1979;9:273–80. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 22.Koslowsky M, Scheinberg Z, Bleich A, et al. The factor structure and criterion validity of the short form of the Eating Attitudes Test. J Pers Assess. 1992;58:27–35. doi: 10.1207/s15327752jpa5801_3. [DOI] [PubMed] [Google Scholar]
- 23.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 24.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17:338–45. [PubMed] [Google Scholar]
- 25.National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. NIH publication no. 98-4083. Bethesda, MD: US Department of Health and Human Services; 1998. [Google Scholar]
- 26.National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) II-A-2. Washington, DC: US Department of Health and Human Services; 2002. [Google Scholar]
- 27.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 28.Landers PS, Landers TL. Survival analysis of dropout patterns in dieting clinical trials. J Am Diet Assoc. 2004;104:1586–8. doi: 10.1016/j.jada.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Cook AJ, Friday JE. Beltsville, MD: USDA, ARS, Community Nutrition Research Group; 2004. [accessed 2 April 2007]. Pyramid servings database for USDA survey food codes. Version 2.0. Internet: http://www.ba.ars.usda.gov/cnrg/ index.html. [Google Scholar]
- 30.Rolls B, Barnett RA. Volumetrics. New York, NY: HarperCollins Publishers, Inc; 2000. [Google Scholar]
- 31.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 32.Baranowski T. How individuals, environments, and health behavior interact: social cognitive theory. In: Glanz K, Rimer BK, Marcus LF, editors. Health behavior and health education: theory, research, and practice. 3. San Francisco, CA: Jossey-Bass; 2002. pp. 165–84. [Google Scholar]
- 33.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: convergent validity. Sports Med. 2002;32:795–808. doi: 10.2165/00007256-200232120-00004. [DOI] [PubMed] [Google Scholar]
- 35.Kyle U, Bosaeus I, De Lorenzo A, et al. ESPEN guidelines. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics. Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey, NHANES III (1998-1994) Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 37.US Department of Health and Human Services. NHANES III anthropometric procedures [videotape] Washington, DC: Public Health Services; 1996. [Google Scholar]
- 38.Ledikwe JH, Blanck HM, Kettel-Khan L, et al. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr. 2005;135:273–8. doi: 10.1093/jn/135.2.273. [DOI] [PubMed] [Google Scholar]
- 39.Womble L, Wadden T, Chandler J, Martin A. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40:131–5. doi: 10.1016/s0195-6663(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 40.Wadden TA, Stunkard AJ, Day SC, Gould RA, Rubin CJ. Less food, less hunger: reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Int J Obes. 1987;11:239–49. [PubMed] [Google Scholar]
- 41.Foster GD, Wadden TA, Peterson FJ, Letizia KA, Bartlett SJ, Conill AM. A controlled comparison of three very-low-calorie diets: effects on weight, body composition, and symptoms. Am J Clin Nutr. 1992;55:811–7. doi: 10.1093/ajcn/55.4.811. [DOI] [PubMed] [Google Scholar]
- 42.Ello-Martin JA, Ledikwe JH, Miller CK, Rolls BJ. Measuring diet satisfaction: the development of a reliable assessment tool. Obes Res. 2004;12:A93. [Google Scholar]
- 43.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition, and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang CS, Alaupovic P, Gregg RE, Brewer HB., Jr Studies on the mechanism of hypertriglyceridemia in Tangier disease. Determination of plasma lipolytic activities, k1 values and apolipoprotein composition of the major lipoprotein density classes. Biochim Biophys Acta. 1987;920:9–19. doi: 10.1016/0005-2760(87)90305-5. [DOI] [PubMed] [Google Scholar]
- 45.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 46.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 47.Gamboa-Pinto AJ, Rock CL, Ferruzzi MG, Schowinsky AB, Schwartz SJ. Cervical tissue and plasma concentrations of alpha-carotene and beta-carotene in women are correlated. J Nutr. 1998;128:1933–6. doi: 10.1093/jn/128.11.1933. [DOI] [PubMed] [Google Scholar]
- 48.Joint National Committee on Prevention. Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 49.Brown H, Prescott R. Applied mixed models in medicine Chichester. United Kingdom: Wiley & Sons, LTD; 1999. [Google Scholar]
- 50.Mallinckrodt CH, Sanger TM, Dube S, et al. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biol Psychiatry. 2003;53:754–60. doi: 10.1016/s0006-3223(02)01867-x. [DOI] [PubMed] [Google Scholar]
- 51.Rock CL, Thornquist MD, Kristal AR, et al. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the sentinel site of the Olestra Post-Marketing Surveillance Study. J Nutr. 1999;129:855–64. doi: 10.1093/jn/129.4.855. [DOI] [PubMed] [Google Scholar]
- 52.Yu-Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris-Etherton PM. Effects of the National Cholesterol Education Program’s Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta-analysis. Am J Clin Nutr. 1999;69:632–46. doi: 10.1093/ajcn/69.4.632. [DOI] [PubMed] [Google Scholar]
- 53.Astrup A, Grunwald GK, Melanson EL, Saris WH, Hill JO. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord. 2000;24:1545–52. doi: 10.1038/sj.ijo.0801453. [DOI] [PubMed] [Google Scholar]
- 54.Kral TVE, Rolls BJ. Energy density and portion size: their independent and combined effects on energy intake. Physiol Behav. 2004;82:131–8. doi: 10.1016/j.physbeh.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 55.Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obes Res. 2001;9:171–8. doi: 10.1038/oby.2001.18. [DOI] [PubMed] [Google Scholar]
- 56.Ledikwe JH, Blanck HM, Khan LK, et al. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006;1006:1172–80. doi: 10.1016/j.jada.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Manson JE, Lee IM, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr. 2000;72:922–8. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 58.Flood A, Velie EM, Chaterjee N, et al. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75:936–43. doi: 10.1093/ajcn/75.5.936. [DOI] [PubMed] [Google Scholar]
- 59.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States) Cancer Causes Control. 2005;16:225–33. doi: 10.1007/s10552-004-4025-1. [DOI] [PubMed] [Google Scholar]
- 61.Eikenberry N, Smith C. Healthful eating: perceptions, motivations, barriers, and promoters in low-income Minnesota communities. J Am Diet Assoc. 2004;104:1158–61. doi: 10.1016/j.jada.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 62.Tsai AC, Sandretto A, Chung YC. Dieting is more effective in reducing weight but exercise is more effective in reducing fat during the early phase of a weight-reducing program in healthy humans. J Nutr Biochem. 2003;14:541–9. doi: 10.1016/s0955-2863(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 63.Lynch NA, Nicklas BJ, Berman DM, Dennis KE, Goldberg AP. Reductions in visceral fat during weight loss and walking are associated with improvements in VO(2 max) J Appl Physiol. 2001;90:99–104. doi: 10.1152/jappl.2001.90.1.99. [DOI] [PubMed] [Google Scholar]
- 64.Ryan AS, Nicklas BJ, Berman DM, Dennis KE. Dietary restriction and walking reduce fat deposition in the midthigh in obese older women. Am J Clin Nutr. 2000;72:708–13. doi: 10.1093/ajcn/72.3.708. [DOI] [PubMed] [Google Scholar]
- 65.Trabulsi J, Schoeller D. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab. 2001;281:E891–9. doi: 10.1152/ajpendo.2001.281.5.E891. [DOI] [PubMed] [Google Scholar]
- 66.Caan B, Ballard-Barbash R, Slattery M, et al. Low energy reporting may increase in intervention participants enrolled in dietary intervention trials. J Am Diet Assoc. 2004;104:357–66. doi: 10.1016/j.jada.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 67.Kristal A, Andrilla C, Koepsell T, Diehr P, Cheadle A. Dietary assessment instruments are susceptible to intervention-associated response set bias. J Am Diet Assoc. 1998;98:40–3. doi: 10.1016/S0002-8223(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 68.Maskarinec G, Robbins C, Riola B, Kane-Sample L, Franke AA, Murphy S. Three measures show high compliance in a soy intervention among premenopausal women. Am J Clin Nutr. 2003;103:861–6. doi: 10.1016/s0002-8223(03)00377-8. [DOI] [PubMed] [Google Scholar]
- 69.Windhauser MM, Evans MA, McCullough ML, et al. Dietary adherence in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S76–83. doi: 10.1016/s0002-8223(99)00420-4. [DOI] [PubMed] [Google Scholar]
- 70.Fitzwater SL, Weinsier RL, Wooldridge NH, Birch R, Liu C, Bartolucci AA. Evaluation of long-term weight changes after a multidisciplinary weight control program. J Am Diet Assoc. 1991;91:421–6. 429. [PubMed] [Google Scholar]
- 71.Weinsier RL, Johnston MH, Doleys DM, Bacon JA. Dietary management of obesity: evaluation of the time-energy displacement diet in terms of its efficacy and nutritional adequacy for long-term weight control. Br J Nutr. 1982;47:367–79. doi: 10.1079/bjn19820048. [DOI] [PubMed] [Google Scholar]
- 72.Greene LF, Malpede CZ, Henson CS, Hubbert KA, Heimburger DC, Ard JD. Weight maintenance 2 years after participation in a weight loss program promoting low-energy density foods. Obesity. 2006;14:1795–801. doi: 10.1038/oby.2006.207. [DOI] [PubMed] [Google Scholar]
- 73.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:S98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]


