Abstract
Oxidative stress is thought to play a role in the pathogenesis of Alzheimer's disease (AD) and increased oxidative DNA damage has been observed in brain tissue from AD patients. Base excision repair (BER) is the primary DNA repair pathway for small base modifications such as alkylation, deamination and oxidation. In this study, we have investigated alterations in the BER capacity in brains of AD patients. We employed a set of functional assays to measure BER activities in brain tissue from short post-mortem interval autopsies of 10 sporadic AD patients and 10 age-matched controls. BER activities were also measured in brain samples from 9 amnestic mild cognitive impairment (MCI) subjects. We found significant BER deficiencies in brains of AD patients due to limited DNA base damage processing by DNA glycosylases and reduced DNA synthesis capacity by DNA polymerase β. The BER impairment was not restricted to damaged brain regions and was also detected in the brains of amnestic MCI patients, where it correlated with the abundance of neurofibrillary tangles. These findings suggest that BER dysfunction is a general feature of AD brains which could occur at the earliest stages of the disease. The results support the hypothesis that defective BER may play an important role in the progression of AD.
INTRODUCTION
Alzheimer's disease (AD) is a progressive age-dependent neurodegenerative disease that leads to cognitive and behavioral impairment. Recent studies show that tissue samples from AD patients have elevated levels of oxidative DNA damage (1–5). A high level of DNA damage can be particularly deleterious in post-mitotic cells because they do not self-renew through cell proliferation. Therefore, oxidative base modifications in nuclear and mitochondrial DNA could lead to selective loss of damaged neurons and may play a significant role in aging and neurodegeneration in mammals (6–8). At present, it is unclear how and why oxidative DNA damage increases in tissues of AD patients; it is also not known whether DNA repair and/or the response to DNA damage play significant roles in the pathogenesis of AD.
Base excision repair (BER) is the primary DNA repair pathway for small base modifications such as alkylation, deamination and oxidation, and is thought to play a critical role during development and maintenance of the central nervous system (CNS) (9). The first step of BER is the removal of the damaged base by a substrate-specific DNA glycosylase, generating an abasic (AP) site, which is cleaved by an AP lyase or AP endonuclease (i.e. APE1 in human cells). In the most common BER sub-pathway, known as short patch BER, the resulting one base gap is filled in by a DNA polymerase and ligated by a DNA ligase. If the 5′ terminal contains blocking groups, the DNA polymerase can add between 2 and 8 nt, with consequent strand displacement, flap processing and finally ligation. This pathway is known as long-patch BER. In humans, DNA polymerase beta is the major DNA polymerase in both sub-pathways (10).
Previous studies of BER in AD patients suggested possible changes in expression of BER enzymes. For example, expression of the mitochondrial β-8-oxoG DNA glycosylase (β-OGG1) was reduced in neuronal cytoplasm of affected AD tissue, and was associated with neurofibrillary tangles (NFT), dystrophic neuritis and reactive astrocytes (11). Reduced expression of DNA polymerase β (pol β) was reported in midtemporal cortex samples from AD patients (12); in contrast, expression of APE1 was higher in affected brain tissue (13) and in extracts of brain cells from AD patients (14). The significance of these observations is not yet known.
This study examines BER capacity in brain tissue from sporadic AD patients and normal age-matched controls. BER activities were also assessed in brain tissue from patients with amnestic mild cognitive impairment (MCI), a syndrome associated with a high risk for the development of dementia and AD (15). The results indicate that AD is associated with a significant impairment of BER function. The BER impairment was not restricted to damaged brain regions and was also detected in the brains of amnestic MCI patients, where it correlated with NFT pathology, a hallmark of AD and related disorders (16).
MATERIALS AND METHODS
Diagnosis of human cases
All patients and controls in this study were longitudinally followed with annual neuropsychological testing and physical and neurological examinations. Some late stage AD patients were not tesee in the final phase of their disease. All controls had neuropsychological test scores in the normal range prior to death. The clinical diagnosis of amnestic MCI was made by consensus conference and followed the criteria of Petersen and Morris (17). The clinical diagnosis of AD followed the standard accepted criteria (18). All AD patients met the National Institute on Aging—Reagan Institute high likelihood guidelines for the neuropathological diagnosis of Alzheimer's disease (19) after histological and immunohistochemical evaluation of 30 different brain regions.
Preparation of brain tissue lysates
Brain specimens used in this study were obtained from short post-mortem interval (PMI) autopsies of 10 AD (six males, four females), 9 amnestic MCI (two males, seven females) and 10 age-matched normal control subjects (six males, four female). Subject demographic data are shown in Table 1. We compared BER activities in affected and unaffected brain regions of AD and control subjects by examination of inferior parietal lobule (IPL) (affected) and cerebellum (CE) (least affected) regions of individual brains. Specimens of IPL and CE were flash frozen in liquid nitrogen at the time of autopsy. Immediately adjacent sections were fixed in 4% formaldehyde for routine histological and immunohistochemical studies. Human brain specimens were suspended in buffer (0.3 g/ml) containing 20 mM HEPES, pH 7.5, 50 mM KCl, 2 mM EGTA and Complete™ protease inhibitor (Roche Applied Sciences, Indianapolis, IN, USA). Tissues were homogenized using a Brinkman Polytron homogenizer for 20 s at setting 4. Lysates were centrifuged at 800 g for 10 min to remove large cell debris. The resulting lysates were resuspended (2 mg/ml) in 20 mM HEPES (pH 7.0), 150 mM KCl, 2 mM EGTA, 1% (w/v) CHAPSO (Sigma), and protease inhibitor mixture and incubated at 4°C for 1 h with end-over-end rotation. The lysates were centrifuged at 100 000 g for 1 h, and the supernatants were collected. The samples were flash frozen in liquid nitrogen and stored at −80°C. Protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA).
Table 1.
Subjects demographic data
| Subjects | Age | Gender | PMI (h) | Neuritic plaques | Braak stage | Cause of death |
|---|---|---|---|---|---|---|
| Control | ||||||
| 1 | 85 | Male | 2 | 5.6 | 3 | Unknown |
| 2 | 86 | Female | 2.25 | 7.6 | 2 | Unknown |
| 3 | 91 | Female | 4 | 10.4 | 1 | Unknown |
| 4 | 86 | Female | 3.75 | 7.8 | 1 | Cardiovascular disease |
| 5 | 81 | Male | 2 | 13.4 | 2 | Pulmonary embolism |
| 6 | 87 | Male | 2.4 | 0.2 | 2 | Prostate cancer |
| 7 | 82 | Male | 2.1 | 1.2 | 1 | Congestive heart failure, pneumonia |
| 8 | 74 | Male | 4 | 0 | 1 | Congestive heart failure |
| 9 | 76 | Female | 2 | 0 | 1 | Chronic obstructive pulmonary disease |
| 10 | 79 | Male | 1.75 | 16.2 | 2 | Bladder cancer |
| Mean ± S.D. | 82.7 ± 5.3 | 2.6 ± 0.9 | 6.2 ± 5.9 | |||
| Alzheimer's | ||||||
| 1 | 83 | Male | 4 | 24.6 | 6 | Aspiration pneumonia |
| 2 | 86 | Female | 4.25 | 23.4 | 6 | Bowel obstruction |
| 3 | 78 | Male | 3.75 | 34.2 | 6 | Unknown |
| 4 | 90 | Female | 2.6 | 30.4 | 6 | Unknown |
| 5 | 75 | Female | 2.33 | 19 | 6 | Congestive heart failure |
| 6 | 81 | Male | 3 | 17.4 | 6 | Unknown |
| 7 | 86 | Female | 3.25 | 19.4 | 6 | Respiratory infection |
| 8 | 74 | Male | 3 | 27.2 | 6 | Fall |
| 9 | 84 | Male | 4.5 | 34.8 | 6 | Unknown |
| 10 | 84 | Male | 2.75 | 31.4 | 6 | Aspiration pneumonia |
| Mean ± S.D. | 82.1 ± 4.8 | 3.3 ± 0.7 | 26.2 ± 6.4 | |||
| Amnestic MCI | ||||||
| 1 | 92 | Female | 3 | 4 | 2 | Aspiration pneumonia |
| 2 | 97 | Female | 2.75 | 10 | 3 | Heart disease |
| 3 | 91 | Female | 5 | 14.2 | 3 | Heart disease |
| 4 | 93 | Female | 2.75 | 11.4 | 3 | Colon cancer |
| 5 | 87 | Male | 3.5 | 22 | 4 | Cardio-pulmonary arrest |
| 6 | 87 | Male | 2.25 | 5 | 3 | Congestive heart failure |
| 7 | 88 | Female | 2.25 | 21.6 | 5 | MI-coronary artery disease |
| 8 | 82 | Female | 3 | 16.2 | 3 | Pulmonary embolism |
| 9 | 99 | Female | 2 | 4 | 5 | Congestive heart failure |
| Mean ± S.D | 90.7 ± 5.31 | 2.9 ± 0.9 | 12.0 ± 7.03 |
Oligonucleotides
The sequences of the oligonucleotides used in this study are presented in Table 2. Oligonucleotides containing 8-oxodG, deoxy-uracil or tetrahydrofuran (THF) (Midland Certified Reagent Company, Midland, TX and Integrated DNA Technologies, Coralville, IA, USA) were 5′-32P-labeled by incubating with [γ-32P] ATP (PerkinElmer, Boston, MA, USA) in the presence of T4 polynucleotide kinase. Unincorporated free [γ-32P] ATP was separated from the reaction mixtures using G25 desalting columns (GE Healthcare Corp., Piscataway, NJ, USA). The 32P-labeled oligonucleotides were then annealed to the complementary strands in the presence of 100 mM KCl by heating the samples at 90°C for 5 min and allowing them to slowly cool to room temperature. For gap-filling reaction and repair synthesis incorporation, unlabeled substrates were annealed as described above.
Table 2.
Names and sequences of oligonucleotides used in this study
| Assay | Name | Sequence |
|---|---|---|
| 8-Oxoguanine incision | OG | 5′-GAA CGA CTG T(OG)A CTT GAC TGC TAC TGA T |
| 3′-CTT GCT GAC A C T GAA CTG ACG ATG ACT A | ||
| Uracil incision and BER synthesis incorporation | UU | 5′-ATA TAC CGC GG(U) CGG CCG ATC AAG CTT ATT |
| 3′-TAT ATG GCG CC G GCC GGC TAG TTC GAA TAA | ||
| AP-site incision | AP | 5′-GAA CGA CTG T (F) A CTT GAC TGC TAC TGA T |
| 3′-CTT GCT GAC A C T GAA CTG ACG ATG ACT A | ||
| Gap-filling | GAP | 5′-CTG CAG CTG ATG CGC ()GT ACG GAT CCC CGG GTA C |
| 3′-GAC GTC GAC TAC GCG GCA TGC CTA GGG GCC CAT G |
OG = 8-oxoguanine; F = tetrahydrofuran abasic site analog.
Oligonucleotide incision assays
8-OxodG incision activity was measured using an oligonucleotide incision assay, as previously described (20). The protein concentration of the lysates for all DNA glycosylase assays was adjusted with 20 mM HEPES–KOH (pH 7.4), 1 mM EDTA, 100 mM KCl, 25% glycerol (v/v), 0.015% Triton X-100, 5 mM DTT and protease inhibitors. Incision reactions (20 μl volume) contained 40 mM HEPES–KOH, 5 mM EDTA, 1 mM DTT, 75 mM KCl, 10% glycerol, 95 fmol of 32P-labeled duplex oligonucleotide. The reactions were incubated at 32°C for 17 h with 16 μg of tissue lysates. The reaction was terminated by the addition of 1 μl each of the following, 5 mg/ml Proteinase K and 10% SDS, and incubated at 55°C for 30 min. The DNA was ethanol-precipitated by the addition of 1 μg of glycogen, 4 μl of 11 M ammonium acetate, and 63 μl ethanol, pelleted, dried and suspended in formamide dye. The samples were resolved in a denaturing 20% polyacrylamide gel containing 7 M urea. After electrophoresis, the gels were visualized using a Molecular Dynamics Phosphoimager (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). The images were analyzed using ImageQuant 5.2 software (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). Incision activity was calculated as the amount of radioactivity in the band corresponding to the damage-specific cleavage product over the total radioactivity in the lane.
Uracil incision activity was measured using a 30-mer oligonucleotide containing a single uracil at position 12 (Table 1). Incision reactions (20 μl) containing 70 mM HEPES–KOH (pH 7.4), 5 mM EDTA, 1 mM DTT, 75 mM NaCl, 10% glycerol and 5 μg of protein were incubated for 1 h at 37°C. The reactions were terminated and DNA processed as described for the measurement of activities of the other glycosylases.
AP endonuclease 1 (APE1) incision activity was measured using a 28-mer oligonucleotide containing the abasic site analog THF at position 11 (Table 1). Samples were diluted in 10 mM HEPES–KOH (pH 7.4) containing 100 mM KCl. Reactions (10 μl) contained 25 mM HEPES–KOH (pH 7.4), 25 mM KCl, 0.1 mg/ml BSA, 5 mM MgCl2, 10% glycerol, 0.05% Triton X-100 and 25 ng protein. Reactions were incubated for the indicated duration at 37°C and terminated by the addition of formamide dye and heating at 90°C for 10 min. Samples were resolved, visualized and analyzed as described for the measurement of DNA glycosylase activities.
Gap-filling assay
Pol β single nucleotide gap-filling activity was measured using a non-labeled 34-mer duplex oligonucleotide containing a single gap at position 16 (Table 1). Samples were diluted in 10 mM Tris–HCl (pH 7.4) containing 100 mM KCl. Reactions (10 μl) contained 50 mM Tris–HCl (pH 7.4), 50 mM KCl, 1 mM DTT, 5 mM MgCl2, 5% glycerol, 5 μM dCTP (Roche Applied Sciences, Indianapolis, IN, USA), 1 pmol of duplex gap oligonucleotide, 4 μCi of α32P-dCTP (GE Healthcare Corp., Piscataway, NJ, USA) and 1 μg protein. Reactions were incubated at 37°C for 1 h or the indicated duration and terminated by the addition of formamide dye and heating at 90°C for 10 min. Samples were resolved and visualized as described above.
Base excision repair synthesis incorporation assay
Repair synthesis reactions (10 μl) contained 40 mM HEPES (pH 7.6), 0.1 mM EDTA, 5 mM MgCl2, 0.2 mg/ml BSA, 20 mM KCl, 1 mM DTT, 40 mM phosphocreatine, 100 μg/ml creatine phosphokinase, 2 mM ATP, 40 μM of each dATP, dTTP, dGTP and 4 μM of dCTP, 0.8 μCi α32P-dCTP, 3% glycerol, 80 ng of double-strand U-containing oligonucleotide and 10 μg tissue lysate protein. The reactions were incubated at 37°C for 3 h and terminated by adding 2.5 μg of proteinase K and 0.5 μl of 10% SDS and incubating at 55°C for 30 min. The DNA was precipitated overnight at −20°C after addition of 1 μg glycogen, 4 μl of 11 M ammonium acetate, 60 μl of ethanol. Samples were centrifuged, dried, suspended in 10 μl of formamide loading dye. The gels were resolved and visualized as described earlier. BER activity was quantified as 32P-dCTP signal strength of the product band relative to control sample #1 (relative activity = 1), after subtracting the background of a reaction without protein.
Western analysis
Proteins in tissue lysates (10–20 µg) were separated on 12% Novex® Tris-glycine gels (Invitrogen, Carlsbad, CA, USA) or 12.5% Criterion Tris–HCl gels (Bio-Rad, Hercules, CA, USA), blotted onto a PVDF membrane and blocked for 1 h at room temperature in 5% non-fat dry milk (Bio-Rad, Hercules, CA, USA) in TBST (20 mM Tris—HCl, pH 7.2, 137 mM NaCl, 0.1% Tween-20). Fresh milk-TBST was added with the primary antibody, which was one of the following: rabbit polyclonal anti-UDG (FL-313) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-human APE1 (Trevigen, Gaithersburg, MD, USA), mouse monoclonal anti-human pol β (Trevigen), rabbit polyclonal anti-beta tubulin (Abcam, Cambridge, MA, USA). Detection was performed with ECL+Plus® (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). Blots were quantified using ImageQuant 5.2 software.
Statistical analysis
The results are reported as mean ± SD. Each assay was performed at least twice. Results from control sample #5 and AD sample # 7 in some assays were statistically defined as outliers based on box plots and were therefore excluded from all statistical analyses in this study. The differences among human control and AD or MCI samples were analyzed by the Student's t-test, and a P < 0.05 was considered statistically significant. Correlation coefficients were calculated using Pearson's correlation test. Trend analysis was performed by calculating the linear contrast using the SAS software version 9.1.
RESULTS
Impaired BER activities and lower protein levels in AD inferior parietal lobule
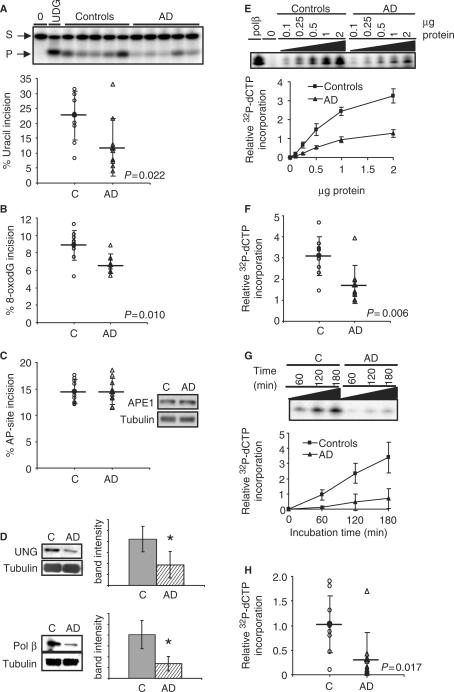
To test whether BER activities are altered in human AD brain, BER assays were conducted using brain tissue from short post-mortem interval autopsies of 10 sporadic AD patients and 10 age-matched human controls (Table 1). The activity of DNA glycosylases was measured as the incision of a radiolabeled DNA oligonucleotide substrate containing a single lesion, either uracil or 8-oxodG. An oligonucleotide containing the abasic site analog THF was used to measure AP-site incision activity. Incision activity was calculated as the amount of radioactivity in the band corresponding to the damage-specific cleavage product over the total radioactivity in the lane. In the inferior parietal lobule (IPL), uracil incision activity was significantly lower (P = 0.022) in AD samples than in control samples (Figure 1A). UDG protein level was also lower in AD samples than in control samples (P < 0.001) (Figure 1D).
Figure 1.
BER activities and protein levels are lower in IPL samples from human AD than in control subjects. (A) Uracil (B) 8-oxodG and (C) AP-site incision activities in IPL lysates of AD and aged-matched control samples. Incision activities were calculated from the amount of radioactivity in the products relative to the total in the lane. (C) Also shows typical western blot analyses of APE1 in AD and control IPL samples. (D) Western blot analyses of UNG and Pol β in AD and control IPL samples. Typical blot image and quantification of all samples is shown. Bar graphs represent average ± SD. * denotes P < 0.05 (E) single nucleotide gap-filling activity as a function of protein concentration in five AD and five control samples. (F) Single nucleotide gap-filling activity of all AD and control samples. (G) Uracil-initiated BER values of five AD and control samples as function of reaction incubation time. (H) Uracil-initiated BER values of all AD and control samples. Mean values are marked by a horizontal line and error bars represent SD.
8-OxodG incision activity was also significantly lower (P = 0.010) in IPL from AD patients (Figure 1B). Since the 8-oxodG DNA glycosylase (OGG1) is the main DNA glycosylase for this lesion in human tissues, these results suggest lower abundance or activity of OGG1 in this tissue from the AD patients. However, AP-site incision activity and protein levels were similar in IPL from AD patients and controls (Figure 1C). Single nucleotide gap-filling capacity in brain tissue was analyzed as the incorporation of a radiolabeled dCTP nucleotide into a 34-mer double-strand substrate containing a single gap. Single nucleotide gap-filling activity was significantly lower (P = 0.006) in IPL from AD patients (Figure 1E and F). Furthermore, pol β protein level was lower in IPL from AD patients than in control samples (P < 0.0001) (Figure 1D).
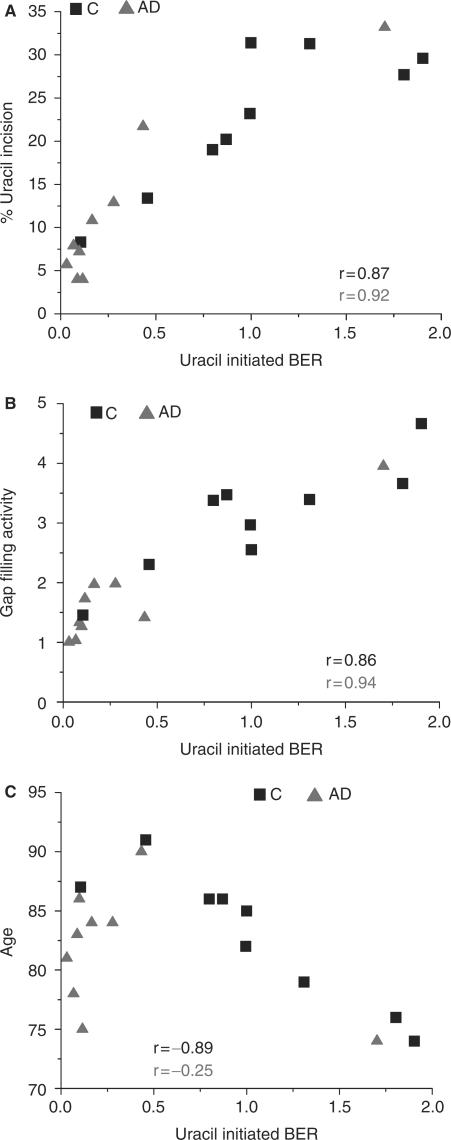
Uracil-initiated BER capacity in brain tissue was analyzed as the incorporation of a radiolabeled dCTP nucleotide into an unlabeled 30-mer double-strand substrate containing a U/G base pair. Because the data presented above indicate that pol β and UDG are reduced in IPL from AD patients, it was predicted that total BER capacity would also be reduced. Indeed, the amount of DNA repair synthesis in a uracil-containing double-stranded oligonucleotide (Table 2) was significantly lower (P = 0.017) in IPL from AD patients than from controls (Figure 1G and H). Total BER capacity positively correlated with UDG activity (r = 0.87 controls; r = 0.92 AD) and pol β activity (r = 0.86 controls; r = 0.94 AD) in control and AD lysates (Figure 2A and B), supporting the idea that the lower BER in the AD lysates was caused by decreased activity of these two enzymes. Furthermore, total BER capacity in controls was inversely correlated with age (r = −0.89), whereas most AD patients had low levels of BER regardless of age (r = −0.25) (Figure 2C).
Figure 2.
Relationship between uracil-initiated BER and either (A) uracil incision activity, (B) gap-filling activity or (C) age in control and AD IPL samples.
Impaired BER activities and lower protein levels in human AD cerebellum
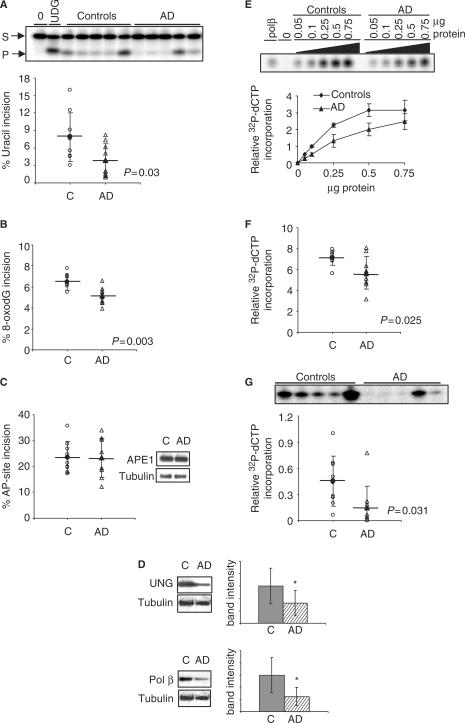
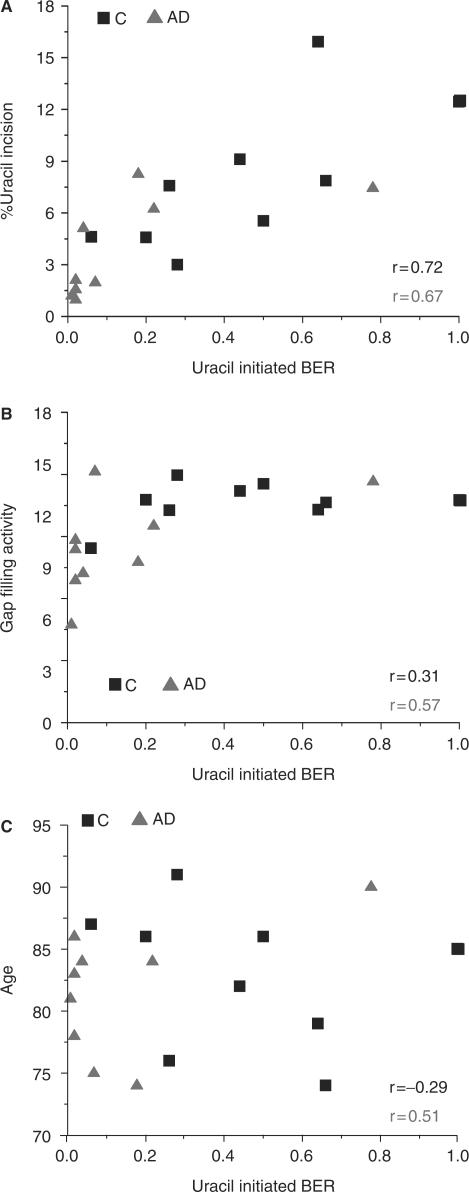
If lower BER activity is a sensitizing feature in AD rather than an underlying cause of the disease, we might expect similar alterations in BER activities in both affected and unaffected regions of brain from AD patients. This question was examined by comparing BER activities in cerebellum (CE) (least affected) and IPL (affected) regions of individual brains. The results indicated that similar changes in BER function occur in CE and IPL brain regions (Figure 3A–G). In particular, uracil incision activity and UDG protein level were significantly reduced (P = 0.030 and P = 0.032, respectively) in AD CE (Figure 3A and D). Moreover, 8-oxoG incision activity was significantly reduced (P = 0.003) in AD samples (Figure 3B). As for the IPL, AP incision activity and APE1 protein level was similar in CE from AD patients and controls (Figure 3C). Additionally, AD CE lysates had significantly reduced (P = 0.025) single nucleotide gap-filling activity (Figure 3E and F) and pol β protein level (P = 0.005) (Figure 3D), although to a lesser extent than IPL. Consequently, total BER capacity was significantly reduced (P = 0.031) in CE from AD patients (Figure 3G). As observed for BER assays in IPL, total BER capacity correlated positively with UDG (r = 0.72 controls; r = 0.67 AD) and pol β (r = 0.31 controls; r = 0.57 AD) (Figure 4A and B) and tended to decrease with the age of controls (r = −0.29), but not with the age of AD patients (Figure 4C).
Figure 3.
BER activities and protein levels are lower in cerebellum samples from human AD than in control subjects. (A) Uracil (B) 8-oxodG and (C) AP-site incision activities in cerebellum lysates of AD and aged-matched control samples. Incision activities were calculated from the amount of radioactivity in the products relative to the total in the lane. (C) Also contains typical western blot analyses of APE1 in AD and control cerebellum samples. (D) Western blot analyses of UNG and Pol β in AD and control IPL samples. Typical blot image and quantification of all samples is shown. Bar graphs represent average ± SD. * denotes P < 0.05 (E) single nucleotide gap-filling activity as a function of protein concentration in five AD and five control samples. (F) Single nucleotide gap-filling activity of all AD and control samples. (G) Gel image of uracil-initiated BER of five AD and control samples, and uracil-initiated BER values of all AD and control samples. Mean values are marked by a horizontal line and error bars represent SD.
Figure 4.
Relationship between uracil-initiated BER and either (A) uracil incision activity, (B) gap-filling activity or (C) age in control and AD cerebellum samples.
Characterization of BER activities in human amnestic MCI inferior parietal lobule
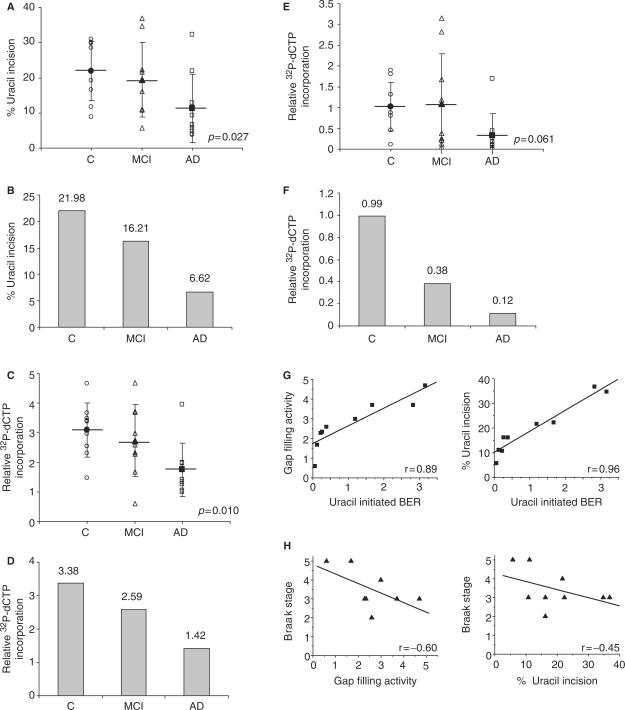
Recent reports showed increased oxidative DNA damage in leukocytes (4) and brain specimens (21) in subjects with amnestic mild cognitive impairment (MCI), a transition phase between normal aging and early dementia and the earliest clinically detectable phase of AD. This suggests that accumulation of DNA damage may be an early event in the progression of AD that could contribute to the pathogenesis of this disease. To test whether loss of BER function occurs in subjects with high risk of developing AD, BER activities were measured in IPL from nine amnestic MCI patients (Table 1), and compared to BER activities in AD patients and controls. The results showed a significant linear trend of decrease in uracil incision activity (P = 0.027) (Figure 5A) and single-nucleotide gap-filling activity (P = 0.010) (Figure 5C) with the severity of the clinical diagnosis. Median uracil incision (Figure 5B) and gap-filling (Figure 5D) activities were 26 and 23% lower in amnestic MCI samples than in controls. Although total BER capacity was not statistically significantly lower in samples from amnestic MCI patients (Figure 5E), median total BER capacity was reduced by 62% (Figure 5F). Uracil incision and gap-filling activities also correlated with total BER capacity in IPL from amnestic MCI patients (Figure 5G).
Figure 5.
BER activities are reduced in IPL samples from human amnestic MCI subjects. (A) uracil incision activities and (B) median uracil incision activity values in amnestic MCI samples compared with controls and AD samples. (C) Single nucleotide gap-filling activities and (D) median gap-filling activity values in amnestic MCI samples compared with controls and AD samples. (E) uracil-initiated BER activities and (F) median uracil-initiated BER activity values in MCI samples compared with controls and AD samples. (G) Relationship between uracil-initiated BER and either gap filling or uracil incision. (H) Relationship between Braak stage and either gap filling or uracil incision activities. Mean values are marked by a horizontal line and error bars represent SD. P-values represent the significance of the linear contrast test.
Amyloid β (Aβ) plaques and NFT are hallmarks of AD. Although there was no correlation between BER activities and the number of Aβ plaques in patients with AD or amnestic MCI (data not shown), BER activities were inversely correlated with Braak stage (22), a measure of NFT abundance, in patients with amnestic MCI (Figure 5H).
DISCUSSION
The goal of this study was to determine whether BER dysfunction plays a role in susceptibility to or progression of AD. This question was addressed by measuring BER activities in brain specimens from patients with AD or normal controls. The results indicate that AD is associated with a significant impairment of general BER function. Our findings show that uracil incision activity and UDG protein levels were significantly lower in brains of AD patients than in controls. Uracil accumulates in DNA as a result of spontaneous deamination of cytosine (23), generating a U:G mismatch; or incorporation of dUMP during replication (24), which results in a U:A base pair. UDG activity decreases rapidly during neuronal development and remains at a low level in adult neurons (25), suggesting that uracil might accumulate in DNA of adult neurons and contribute to neuronal aging (26). Furthermore, a recent study reported that suppression of UDG expression induced apoptosis in cultured rat hippocampal neurons (27), supporting a role for this enzyme in maintaining neuronal viability. Importantly, folic acid deficiency, which has been linked to increased susceptibility to AD (28), promoted uracil misincorporation and hypomethylation of DNA in neurons and sensitized them to Aβ toxicity (29). It also resulted in increased DNA damage and hippocampal neurodegeneration in APP transgenic mice (29). This is consistent with the possibility that reduced uracil incision capacity could sensitize neurons to Aβ toxicity in the brains of AD patients.
8-OxodG incision activity is a primary function of OGG1, a bifunctional DNA glycosylase with a strong glycosylase activity but weak AP lyase activity (30,31). Lower OGG1 activity was previously observed in nuclear lysates from affected human AD brain regions using a sodium borohydride trapping assay (32). This assay detects the covalent complex formed between the AP lyase activity of OGG1 and the abasic site intermediate, and thus measures only the robustness of the AP lyase activity. By employing a DNA cleavage assay we show here that 8-oxodG incision activity of OGG1 is lower in AD extracts independent from its limited AP-lyase activity.
The finding that AP-site incision activity and APE1 protein levels were similar in brains of AD patients and controls differs from previous reports of increased APE1 expression in AD (13,14). However, only expression levels and not APE1 activity was reported in the previous studies. The activity is a more finite determination of function. However, there could be issues with different experimental protocols, such as post-mortem interval and tissue handling.
Single nucleotide gap-filling activity and pol β protein level were also significantly reduced in brains of AD patients. DNA pol β protects cells against the cytotoxicity of oxidative DNA damage (33) and plays a role in genome maintenance in aging and carcinogenesis (34). Importantly, mice lacking pol β display neonatal lethality with abnormal neurogenesis characterized by apoptotic cell death in the developing central and peripheral nervous systems, but not in other tissues (35). A recent report (12) on reduced pol β protein levels in AD brains supports our observation. DNA pol β contributes two essential enzymatic activities to BER: a 5′-deoxyribose phosphate (dRP) lyase activity, necessary to remove the dRP intermediate generated by APE1 cleavage of the abasic site, and a nucleotidyl transferase activity that incorporates the correct nucleoside triphosphate in a template-dependent manner (36). While we have not directly measured dRP-lyase activity in these samples, the observation of decreased gap filling indicates a likely defect in pol β-catalyzed DNA synthesis in brains of AD patients.
Overall BER capacity ultimately determines the efficiency of repair of BER-specific lesions. Our results show that total uracil-initiated BER was significantly lower in brains of patients with AD. Moreover, the finding that total BER capacity correlated positively with UDG and pol β activities in control and AD brains supports the idea that the lower BER was caused by decreased activity of these two enzymes. Notably, total BER capacity was inversely correlated with age of controls, but not with age of AD patients. Instead, the low BER capacity associated with AD regardless of age suggests a premature aging phenotype.
It is important to note that the BER defects reported here were not limited to neuropathologically affected regions of AD brains, but instead were apparent in IPL and CE of AD patients. This suggests that BER dysfunction is a general feature of AD brains. This observation also dissociates the reduced BER levels in the IPL from selective loss of neurons in this region, since there is no neuronal cell death in the cerebellum of AD patients.
MCI is a syndrome defined as cognitive decline greater than the expected for an individual's age and education level but that does not interfere notably with activities of daily life (37). Although some individuals with MCI remain stable or even return to normal over time, more than half progress to dementia within five years. The amnestic subtype of MCI, examined in the present study, has the highest risk of progression to AD. Interestingly, BER activities were reduced in brain tissue from patients with amnestic MCI, a condition also characterized by increased load of oxidative DNA damage (4,21). This suggests that BER dysfunction, and increased accumulation of oxidative DNA damage, could occur at the earliest stages of dementia and AD.
Aβ plaques and NFT are hallmarks of AD. Thus, it is important to ask whether BER dysfunction is associated with these neuropathological features. Gabbita and colleagues (5) found no correlation between the number of oxidative DNA lesions in AD brain regions and the number of NFT or Aβ plaques. Similarly, BER dysfunction did not correlate with the number of Aβ plaques in this study. However, BER activities and NFT were inversely correlated with Braak stage (22), a measure of NFT abundance, in brains of amnestic MCI patients. A similar pattern could not be observed in brains of AD patients because all AD patients in this study were classified in the highest Braak stage (VI). The possible heterogeneity of outcome of amnestic MCI patients supports the finding that the BER deficiency correlates with the NFT pathology. Moreover, since NFT pathology in AD is associated with cognitive decline (38), our finding suggests a link between BER capacity and the degree of neurological impairment, as measured by Braak stage.
The question of how BER deficiency is involved in the progression of AD has yet to be answered. One possibility is that lack of proficient BER sensitizes neurons to the deleterious effects of Aβ and NFT. It has been speculated that a cause for oxidative DNA damage in AD is the accumulation of Aβ itself. This hypothesis resulted from the observation that Aβ can directly generate hydrogen peroxide through iron and copper ion reduction (39,40). The combined effect of increased oxidative DNA damage and a significant deficiency in DNA repair could potentially lead to neuronal loss. This may also explain why although BER deficiency was detected in both affected and non-affected regions of AD brains, neuronal loss is limited to areas where Aβ plaques and NFT are present.
In summary, this study demonstrates significant BER dysfunction in brains of AD patients, resulting from reduced UDG, OGG1 and pol β activities. Our findings that BER deficiencies were detected in both affected and non-affected brain regions of AD patients suggest that impairment of BER is a general feature of AD brains. We also show that BER activities in amnestic MCI patients inversely correlated with the severity of disease. Together, these findings suggest that defective BER may play an important role in the progression of AD. The results presented here may lead to better understanding of the molecular mechanisms involved in AD, and pave the way to the development of risk assessment tools as well as preventive drug therapy.
ACKNOWLEDGEMENTS
We thank Dr Xiufen Sui for her technical assistance; Dr David Wilson III and his lab members for purified proteins and oligonucleotides; Dr Larry Brant for his assistance with the statistical analysis; Dr David Wilson III and Dr Shepherd Schurman for their critical review of this manuscript. This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health and 1P30 AG0 28383. Funding to pay the Open Access publication charges for this article was provided by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Conflict of interest statement. None declared.
REFERENCES
- 1.Kadioglu E, Sardas S, Aslan S, Isik E, Esat KA. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer's disease. Biomarkers. 2004;9:203–209. doi: 10.1080/13547500410001728390. [DOI] [PubMed] [Google Scholar]
- 2.Mecocci P, Polidori MC, Ingegni T, Cherubini A, Chionne F, Cecchetti R, Senin U. Oxidative damage to DNA in lymphocytes from AD patients. Neurology. 1998;51:1014–1017. doi: 10.1212/wnl.51.4.1014. [DOI] [PubMed] [Google Scholar]
- 3.Morocz M, Kalman J, Juhasz A, Sinko I, McGlynn AP, Downes CS, Janka Z, Rasko I. Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer's disease. Neurobiol. Aging. 2002;23:47–53. doi: 10.1016/s0197-4580(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 4.Mighore L, Fontana I, Trippi F, Colognato R, Coppede F, Tognoni G, Nucciarone B, Siciliano G. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol. Aging. 2005;26:567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J. Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 6.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 7.Melov S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 9.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don't divide what's to repair? Mutat. Res. 2006;614:24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Dianov GL, Prasad R, Wilson SH, Bohr VA. Role of DNA polymerase beta in the excision step of long patch mammalian base excision repair. J. Biol. Chem. 1999;274:13741–13743. doi: 10.1074/jbc.274.20.13741. [DOI] [PubMed] [Google Scholar]
- 11.Iida T, Furuta A, Nishioka K, Nakabeppu Y, Iwaki T. Expression of 8-oxoguanine DNA glycosylase is reduced and associated with neurofibrillary tangles in Alzheimer's disease brain. Acta Neuropathol. (Berl) 2002;103:20–25. doi: 10.1007/s004010100418. [DOI] [PubMed] [Google Scholar]
- 12.Copani A, Hoozemans JJ, Caraci F, Calafiore M, Van Haastert ES, Veerhuis R, Rozemuller AJ, Aronica E, Sortino MA, et al. DNA polymerase-beta is expressed early in neurons of Alzheimer's disease brain and is loaded into DNA replication forks in neurons challenged with beta-amyloid. J. Neurosci. 2006;26:10949–10957. doi: 10.1523/JNEUROSCI.2793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Z, Sun N, Schreiber SS. Immunohistochemical localization of redox factor-1 (Ref-1) in Alzheimer's hippocampus. Neuroreport. 1998;9:2749–2752. doi: 10.1097/00001756-199808240-00012. [DOI] [PubMed] [Google Scholar]
- 14.Davydov V, Hansen LA, Shackelford DA. Is DNA repair compromised in Alzheimer's disease? Neurobiol. Aging. 2003;24:953–968. doi: 10.1016/s0197-4580(02)00229-4. [DOI] [PubMed] [Google Scholar]
- 15.Levey A, Lah J, Goldstein F, Steenland K, Bliwise D. Mild cognitive impairment: an opportunity to identify patients at high risk for progression to Alzheimer's disease. Clin. Ther. 2006;28:991–1001. doi: 10.1016/j.clinthera.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC, Morris JC. Clinical features. In: Petersen RC, editor. Miild cognitive impairment; Aging to Alzheimer's disease. Oxford: Oxford University Press; 2003. pp. 15–39. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol. Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 20.Souza-Pinto NC, Croteau DL, Hudson EK, Hansford RG, Bohr VA. Age-associated increase in 8-oxo-deoxyguanosine glycosylase/AP lyase activity in rat mitochondria. Nucleic Acids Res. 1999;27:1935–1942. doi: 10.1093/nar/27.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J. Neurochem. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl T, Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974;13:3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- 24.Tye BK, Lehman IR. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J. Mol. Biol. 1977;117:293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- 25.Focher F, Mazzarello P, Verri A, Hubscher U, Spadari S. Activity profiles of enzymes that control the uracil incorporation into DNA during neuronal development. Mutat. Res. 1990;237:65–73. doi: 10.1016/0921-8734(90)90012-g. [DOI] [PubMed] [Google Scholar]
- 26.Mazzarello P, Focher F, Verri A, Spadari S. Misincorporation of uracil into DNA as possible contributor to neuronal aging and abiotrophy. Int. J. Neurosci. 1990;50:169–174. doi: 10.3109/00207459008987169. [DOI] [PubMed] [Google Scholar]
- 27.Kruman II, Schwartz E, Kruman Y, Cutler RG, Zhu X, Greig NH, Mattson MP. Suppression of uracil-DNA glycosylase induces neuronal apoptosis. J. Biol. Chem. 2004;279:43952–43960. doi: 10.1074/jbc.M408025200. [DOI] [PubMed] [Google Scholar]
- 28.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am. J. Clin. Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 29.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J. Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjoras M, Luna L, Johnsen B, Hoff E, Haug T, Rognes T, Seeberg E. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J. 1997;16:6314–6322. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zharkov DO, Rosenquist TA, Gerchman SE, Grollman AP. Substrate specificity and reaction mechanism of murine 8-oxoguanine-DNA glycosylase. J. Biol. Chem. 2000;275:28607–28617. doi: 10.1074/jbc.M002441200. [DOI] [PubMed] [Google Scholar]
- 32.Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer's disease brain. Brain Res. 2000;855:116–123. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- 33.Chen KH, Yakes FM, Srivastava DK, Singhal RK, Sobol RW, Horton JK, Van HB, Wilson SH. Up-regulation of base excision repair correlates with enhanced protection against a DNA damaging agent in mouse cell lines. Nucleic Acids Res. 1998;26:2001–2007. doi: 10.1093/nar/26.8.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabelof DC, Ikeno Y, Nyska A, Busuttil RA, Anyangwe N, Vijg J, Matherly LH, Tucker JD, Wilson SH, et al. Haploinsufficiency in DNA polymerase beta increases cancer risk with age and alters mortality rate. Cancer Res. 2006;66:7460–7465. doi: 10.1158/0008-5472.CAN-06-1177. [DOI] [PubMed] [Google Scholar]
- 35.Sugo N, Aratani Y, Nagashima Y, Kubota Y, Koyama H. Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase beta. EMBO J. 2000;19:1397–1404. doi: 10.1093/emboj/19.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beard WA, Wilson SH. Structural design of a eukaryotic DNA repair polymerase: DNA polymerase beta. Mutat. Res. 2000;460:231–244. doi: 10.1016/s0921-8777(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 38.Bancher C, Braak H, Fischer P, Jellinger KA. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease patients. Neurosci. Lett. 1993;162:179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, et al. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 40.Opazo C, Huang X, Cherny RA, Moir RD, Roher AE, White AR, Cappai R, Masters CL, Tanzi RE, et al. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2) J. Biol. Chem. 2002;277:40302–40308. doi: 10.1074/jbc.M206428200. [DOI] [PubMed] [Google Scholar]