Abstract
Cough and sniff are both spontaneous respiratory behaviors that can be initiated voluntarily in humans. Disturbances of cough may be life threatening, while inability to sniff impairs the sense of smell in neurological patients. Cortical mechanisms of voluntary cough and sniff production have been predicted to exist; however, the localization and function of supramedullary areas responsible for these behaviors are poorly understood. We used functional magnetic resonance imaging to identify the central control of voluntary cough and sniff compared with breathing. We determined that both voluntary cough and sniff require a widespread pattern of sensorimotor activation along the Sylvian fissure convergent with voluntary breathing. Task-specific activation occurred in a ponto-mesencephalic region during voluntary coughing and in the hippocampus and piriform cortex during voluntary sniffing. Identification of the localization of cortical activation for cough control in humans may help potential drug development to target these regions in patients with chronic cough. Understanding the sensorimotor sniff control mechanisms may provide a new view on the cerebral functional reorganization of olfactory control in patients with neurological disorders.
Keywords: respiratory control, cerebral cortex, functional MRI
Introduction
Coughing and sniffing are innate respiratory behaviors that do not require conscious intervention. Coughing is an airway defensive act to clear mucus and foreign particles from the tracheobrochial tree and lungs by generating large expiratory airflows (Karlsson et al., 1988). Sniffing is a rapid inspiratory behavior aiding olfaction (Mainland and Sobel, 2006). Neurophysiological studies in animals and humans have shown that neural mechanisms of both reflex cough and sniff are regulated by the inspiratory and expiratory networks of the brainstem, pons, and cerebellum (Mainland and Sobel, 2006; Shannon et al., 1998; Xu et al., 1997). Unique to humans, cough and sniff can be activated or suppressed on demand independent of natural sensory stimulation. Performance of these voluntary behaviors is thought to depend upon supramedullary regions for conscious control of respiratory acts. However, the central neural mechanisms involved in voluntary control of cough and sniff remain poorly defined (Mainland and Sobel, 2006; Widdicombe et al., 2006).
Voluntary control of human cough has been observed in experiments of cough initiation and suppression on demand during capsaicin inhalation (Hutchings et al., 1993b) and upper respiratory tract infection (Hutchings et al., 1993a). Down-regulation of cortical cough control has been demonstrated during rapid eye movement sleep and anesthesia with reoccurrence only after arousal from sleep (Jamal et al., 1983; Nishino et al., 1988). It was suggested that the cerebral cortex modulates the sensitivity of reflex cough centers in the brainstem (Lee et al., 2002) by generation of central neuromodulators, such as endogenous opioids (Lee et al., 2005). Voluntary cough can be impaired in patients with stroke in the frontoparietal region and basal ganglia (Addington et al., 1999; Stephens et al., 2003), Parkinson's disease (Fontana et al., 1998), multiple system atrophy (Nishino et al., 2004), and motor neuron disease (Hadjikoutis et al., 2000). In some patients, voluntary cough can be diminished independently from reflex cough suggesting the presence of separate higher control centers for voluntary coughing. For example, following stroke some patients exhibit a loss of voluntary cough with preserved reflex coughing (Addington et al., 1999; Stephens et al., 2003), whereas in Parkinson's disease both voluntary and reflex cough thresholds can be depressed in early manifestation of the disease (Fontana et al., 1998). Voluntary cough dysfunction independent from reflex cough in neurological disorders is presumably due to disruption of facilitation between the putative cortical and brainstem cough centers (Widdicombe and Singh, 2006).
Voluntary control of sniff was investigated as part of olfaction (Sobel et al., 1998a); however, its sensorimotor aspects have received little attention (Mainland and Sobel, 2006). During sniffing for smelling, the brainstem inspiratory neurons interact with the piriform cortex via the hippocampus (Vanderwolf, 2001) and cerebellum (Sobel et al., 1998b). The hippocampus exhibits its olfactomotor control through separate but linked pathways of olfactory and sniffing regulation (Vanderwolf, 2001), whereas the cerebellum optimizes the act of sniffing for olfactory processing (Mainland and Sobel, 2006). Decreased ability to sniff voluntarily has been reported to influence the sense of smell in patients with unilateral cerebellar lesions and neurodegenerative diseases (Abele et al., 2003; Connelly et al., 2003; Mainland et al., 2005; Sobel et al., 2001). However, the sniff-like reflex may be preserved in severe hypoxic coma (Tomori et al., 1991), possibly due to functional disruption between the cortical and brainstem pathways (Mainland and Sobel, 2006).
To understand the supramedullary control of these voluntary respiratory behaviors that have a common brainstem control, we investigated cerebral activation during voluntary coughing, sniffing, and breathing using functional magnetic resonance imaging (fMRI). We hypothesized that production of voluntary cough and sniff will elicit activation in the periSylvian region similar to voluntary breathing control (Ramsay et al., 1993). We expected that voluntary cough and sniff, as complex respiratory behaviors, would recruit larger areas of activation within the same brain regions. We also hypothesized that task-specific activation in distinct cortical and subcortical regions would differentiate between these voluntary respiratory tasks.
Materials and Methods
Subjects
Fifteen healthy volunteers, eight females and seven males (age 33.3 ± 12.3 years, mean ± standard deviation), participated in the study. All subjects were right-handed on the Edinburgh Handedness Inventory (Oldfield, 1971) and monolingual native English speakers. Physical examinations in all participants were normal and none had any history of neurological, psychiatric, or respiratory problems. Video fiberoptic nasolaryngoscopy confirmed normal anatomy of the upper airways and larynx and normal task production in all participants. All subjects provided written informed consent before participation in the study, which was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, NIH.
Tasks
The experimental tasks involved voluntary breathing, coughing, sniffing, and passive breathing as a baseline condition. Prior to the scanning, participants were trained in task production outside of the magnet. Subjects were instructed to listen attentively to the auditory example of the task (e.g., sound of a person coughing, sniffing or breathing voluntarily) and to reproduce it at a comfortable level without maximal but relatively constant respiratory effort. Auditory stimuli were recorded from a female native English speaker for the purpose of the study. For voluntary cough, subjects were instructed to produced two pairs of coughs through the mouth as a brief inspiration followed by forced expiration for each pair with their glottis closed at the beginning of expiration similar to a spontaneous cough (Widdicombe and Fontana, 2006). For voluntary sniff, participants produced two pairs of sniffs as forced inspirations through the nose with their mouth closed. For voluntary breathing, participants produced a prolonged inspiration followed by prolonged expiration through the mouth. A resting condition of passive breathing without any auditory sample presentation served as control.
Experimental design
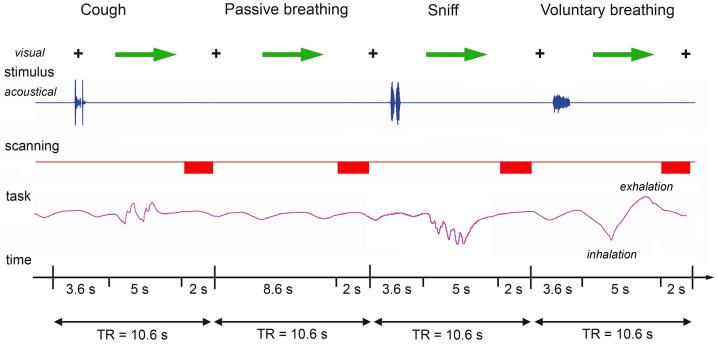
The major limitation in the studies involving orofacial behaviors is the susceptibility to motion-induced artifacts, which may lead to false signal changes and spatial misalignment because of movement-induced field changes and possible head drift along the scanning session (Birn et al., 1999). To reduce these artifacts and to neutralize the scanner noise interference with acoustic stimulus presentation, an event-related clustered volume acquisition fMRI design was used as follows. During the experiment, the subjects were first asked to fixate their attention on the black cross while listening to the same auditory samples introduced through the MR-compatible headphones (Silent Scan™ Audio Systems, Avotec Inc., Stuart, FL) for 3.6-s period. A visual cue (green arrow) then instructed the subjects to reproduce the task within the 5-s interval, which was followed by a 2-s period of image acquisition when the subjects again fixated their attention on the black cross (Fig. 1). Auditory stimulus delivery and motor task production by subjects occurred during the silent period only (8.6 s); therefore, it did not interfere with image acquisition and minimized task-induced signal artifacts. Six scanning sessions were acquired; each session consisted of 36 trials of experimental tasks and 24 resting conditions. All tasks were pseudo-randomized between sessions and subjects. The sounds of subjects' task productions while in the scanner were recorded using an MR-compatible microphone attached to the headphones (Silent Scan™ Audio Systems, Avotec Inc., Stuart, FL). Subjects' respiratory performance was recorded using an MRI-compatible pneumatic belt positioned at the level of the abdomen to monitor the accuracy of task performance. Changes in air pressure within the belt were observed during respiratory movements; however, because of the difficulty in maintaining a constant position of the belt during scanning session, these signals were not accurate for measuring respiratory volume or flow during the task production.
Figure 1. Schematic illustration of the experimental design in a single subject.
The subject fixated on the black cross and listened to the acoustically presented sample task for a 3.6-s period. Samples were pseudorandomized and presented as pairs of voluntary coughing and sniffing and a single voluntary breathing. No stimulus was presented for passive breathing (control condition), during which the subject maintained normally paced breathing. A green arrow cued the subject to initiate the task production within a 5-s period, which was followed by a 2-s period of image acquisition. Respiratory movements during production of all tasks were recorded in arbitrary units to monitor the correct task performance.
Data acquisition
All images were acquired on a 3.0 Tesla whole-body scanner equipped with a quadrature birdcage radio frequency head coil (Signa, General Electric Medical Systems, Milwaukee, WI). To restrain head movements, the subject's head was comfortably immobilized using a forehead strap and a vacuum pillow filled with polystyrene balls. Functional images were acquired with a gradient-weighted echo planar imaging (EPI) pulse sequence (TE = 30 ms; TR = 2 s per volume, 10.6 s between volumes; FA = 90 degrees; FOV = 240 mm; matrix 64 × 64 mm; in-plane resolution 3.75 mm) using blood oxygenation level-dependent (BOLD) contrast. The whole brain coverage was achieved with 35 sagittal slices with a slice thickness of 4 mm without gap during each imaging run. High-order shimming prior to the EPI acquisition optimized the homogeneity of the magnetic field across the brain and minimized EPI distortions. A high-resolution T1-weighted anatomical image was collected using 3D inversion recovery prepared spoiled gradient-recalled sequence (3D IR-Prep SPGR; TI = 450 ms; TE = 3.0 ms; FA = 12 degrees; bandwidth = 31.25 mm; FOV = 240 mm; matrix 256 × 256 mm; 128 contiguous axial slices; slice thickness 1.0 mm; slice spacing 1.0 mm).
Data analysis
Functional imaging data were analyzed using the AFNI software package (http://afni.nimh.nih.gov) (Cox, 1996). For each subject, the first two volumes in each series, collected before equilibrium magnetization was reached, were discarded. The EPI volumes were registered to the volume collected closest in time to the high-resolution anatomical scan using heptic polynomial interpolation, spatially smoothed with a 4-mm Gaussian filter, and then scaled by the mean signal change at each voxel. The task-related responses were analyzed using multiple linear regression with a single regressor for each task convolved with a canonical hemodynamic response function. Baseline drifts were modeled using quadratic polynomials in time for each separate imaging run, and motion parameter estimates were used as additional regressors of no interest in the multiple regression analysis. The correction for multiple comparisons was made using Monte-Carlo simulations (Forman et al., 1995) that resulted in a voxelwise threshold of 0.001 and a minimum cluster size of eight contiguous voxels at a corrected p value of 0.034. For group analysis, the 3-D anatomical datasets of each subject were spatially normalized and converted to the standard anatomical space of Talairach and Tournoux (Talairach and Tournoux, 1988). The resulting normalization parameters were applied to the 4-D time series datasets, which were transformed into standard stereotaxic space. To estimate the main effect of voluntary cough, sniff and breathing, the group analysis was carried out using a two-way within-subject mixed effects design analysis of variance (ANOVA) with subject as the random factor and the task as the fixed factor (p ≤ 0.05, corrected). Conjunction analyses were performed to determine regions of overlapping and distinct activation during voluntary cough/voluntary breathing, voluntary sniff/voluntary breathing, and voluntary cough/voluntary sniff, respectively (voxel probability threshold p = 0.001; minimum cluster size of 8 voxels, 450 mm3, volume of 1 EPI voxel at original resolution is 56.25 mm3).
For region-of-interest (ROI) analyses, anatomical parcellations to define the primary motor cortex (areas 4a and 4p) (Geyer et al., 1996), primary sensory cortex (areas 3a, 3b, 1, and 2) (Geyer et al., 1999; Geyer et al., 2000; Grefkes et al., 2001), premotor cortex (area 6) (Geyer, 2003), secondary somatosensory cortex (OP 1-4) (Eickhoff et al., 2006a; Eickhoff et al., 2006b), ventral thalamus, and striatum were based on the maximum probability maps (MPM) and macrolabels maps (Eickhoff et al., 2005). For each subject, voxels with positive values were identified using a threshold of p = 0.001 and a minimum cluster size of eight contiguous voxels that were at least 3.8 mm apart within a ROI. The total volume of active voxels and mean percent BOLD signal change were extracted per ROI in each subject. Prior to ROI analysis, relationships between the mean percent activation volume and mean percent BOLD signal change during voluntary coughing, sniffing and breathing were examined using Pearson's correlation coefficients. Because these two measures were highly correlated during breathing (r = 0.961, p = 0.0005), coughing (r = 0.926, p = 0.005), and sniffing (r = 0.976, p = 0.0005), the ANOVA estimated the influence of task using mean percent activation volume only. The three-factor ANOVA examined task (cough vs. breathing, sniff vs. breathing, cough vs. sniff), ROI (primary motor, premotor, primary sensory, secondary sensory, subcortical regions), and hemispheric laterality (right vs. left) effects between voluntary cough/voluntary breathing, voluntary sniff/voluntary breathing, and voluntary cough/voluntary sniff, respectively (p ≤ 0.05). If the main effect for task was significant, individual ROIs were evaluated for the task effect and task interactions with hemispheric laterality (p ≤ 0.025).
Results
Behavioral measurements
Respiratory movement signals, recorded during each scanning session, demonstrated that all subjects performed the tasks correctly in response to the audio-visual cues. The passive breathing pace was relatively consistent during the rest condition within a subject.
Common activation across voluntary cough, sniff, and breathing
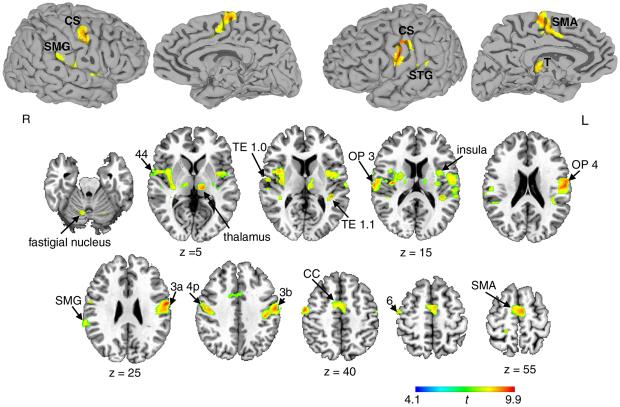
Voluntary cough, sniff and breathing elicited overlapping activation in both hemispheres when contrasted with passive breathing. A large cluster of bilateral activation included the precentral (areas 4a, 4p, and 6) and postcentral (areas 3a, 3b, 2, and 1) gyri extending to the inferior frontal gyrus (area 44) rostrally, to the operculum (OP 1-4) and insula ventrally, and to the superior temporal and supramarginal gyri caudally. On the medial surface, bilateral cortical activation occurred in the supplementary motor area (SMA-proper) extending to the anterior and middle cingulate cortex. Subcortical activation was found in the ventral and dorsomedial thalamus, caudate nucleus, putamen, and lateral globus pallidus. Cerebellar activation involved the declive and culmen in both hemispheres and the right fastigial nucleus (Fig. 2; see also Supplementary Table 1).
Figure 2. Common activation during voluntary cough, sniff and breathing relative to passive breathing.
Spatially normalized activation was registered and projected onto the single-subject template in the Talairach-Tournoux standard space (group mean activation, p ≤ 0.05, corrected). The color scale illustrates t-values (14 degrees of freedom). CS – central sulcus; SMG – supramarginal gyrus; STG – superior temporal gyrus; SMA – supplementary motor area; OP – operculum; CC – cingulate cortex; T – thalamus; R –right; L - left.
Comparison between voluntary cough and voluntary breathing
In addition to the regions of common activation described above, conjoint activation during cough and voluntary breathing occurred in the substantia nigra and the dentate nucleus of the cerebellum bilaterally. Voluntary cough only elicited significant activation in the left precuneus and the lingual and inferior temporal gyri bilaterally. Subcortically, coughing activated the amygdala, lateral thalamus and subthalamic nucleus bilaterally, extending caudally into the ponto-mesencephalic region. Cerebellar activation was spread over both hemispheres across the vermis (Fig 3).
Figure 3. Group common and distinct activation during voluntary coughing and voluntary breathing.
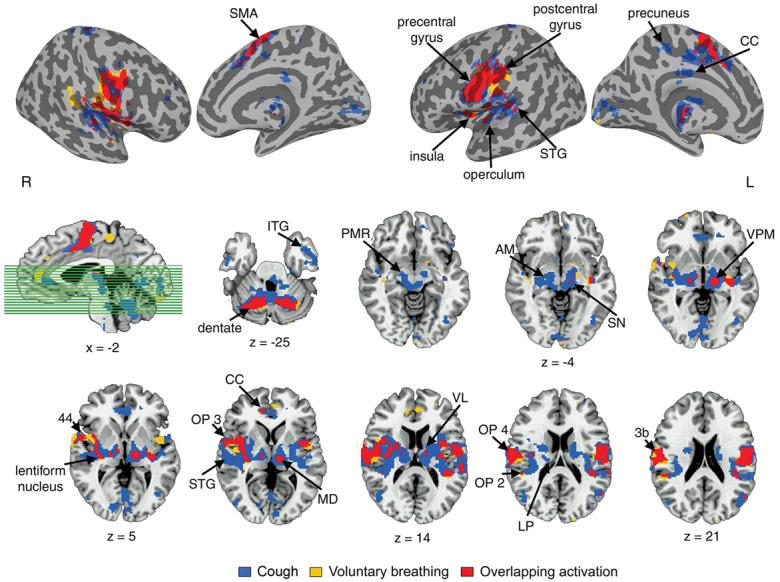
Mean cortical activation is superimposed onto the inflated cortical surfaces; activation in the subcortical regions is presented in the series of brain images in axial view of the single subject template in the Talairach-Tournoux standard space (cluster size ≥ 8 contiguous voxels; voxel threshold p = 0.001). The sagittal image shows the location of the axial cut-planes. Plane coordinates are displayed, respectively. SMA – supplementary motor area; CC – cingulate cortex; STG – superior temporal gyrus; ITG – inferior temporal gyrus; PMR – ponto-mesencephalic region; AM – amygdala; SN – substantia nigra; VPM – ventral posteromedial nucleus; VL – ventrolateral nucleus; MD – mediodorsal nucleus; OP – operculum; LP – lateral posterior thalamus; L - left hemisphere; R - right hemisphere.
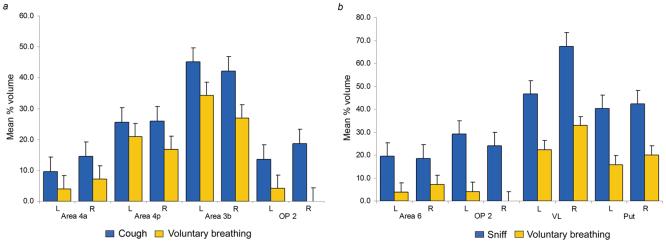
Voluntary cough production resulted in markedly greater volume of overall brain activation (total volume of 14,060 mm3) compared with voluntary breathing (6,587 mm3). The region-of-interest (ROI) analysis of mean percent activation volume identified a significant task effect (F1,14 = 9.167, p = 0.009) with significant task by ROI (F12,168 = 6.544, p = 0.0005) and task by ROI by hemispheric laterality (F12,168 = 4.087, p = 0.0005) interactions. A significant percent activation volume increase during voluntary coughing compared with voluntary breathing occurred within the primary motor cortex (area 4a: F1,14 = 6.391, p = 0.024; area 4p: F1,14 = 11.158, p = 0.005), primary somatosensory cortex (area 3b: F1,14 = 9.367, p = 0.008), and in the parieto-opercular cortex (OP 2: F1,14 = 5.611, p = 0.013) (Fig. 5a).
Figure 5. Region-of interest analyses comparing voluntary coughing with voluntary breathing and voluntary sniffing with voluntary breathing.
(a) Coughing compared with voluntary breathing elicited significant increases in mean percent activation volume in the primary motor cortex (p ≥ 0.024), primary somatosensory cortex (p = 0.008) and parietal operculum (p = 0.013). (b) Sniffing compared with voluntary breathing produced significant increases in mean percent activation volume in the premotor cortex (p = 0.016), parietal operculum (p =0.020), ventrolateral thalamus (p = 0.008) and the putamen (p =0.005). Error bars report the standard error. OP – operculum; VL – ventrolateral nucleus; Put – putamen; L -left hemisphere; R - right hemisphere; n = 15 subjects for all ROI mean values.
Comparison between voluntary sniff and voluntary breathing
In addition to the regions of common activation observed during all voluntary respiratory tasks, conjoint activation during voluntary sniff and voluntary breathing occurred in the medial globus pallidus and dentate nucleus of the cerebellum. Cortical activation during voluntary sniffing, but not during voluntary breathing, was found in the posterior cingulate cortex, lingual gyrus, piriform cortex, the parahippocampal gyrus and hippocampus proper with its subdivisions Cornu ammonis, dentate gyrus, subiculum, hippocampal-amygdaloid transitional area (HATA) bilaterally, the right precuneus, and the left fusiform gyrus. Subcortical activation was identified bilaterally in the lateral thalamus, subthalamic nucleus, amygdala, and the pons (Fig. 4).
Figure 4. Group conjoint and distinct activation during voluntary sniffing and voluntary breathing.
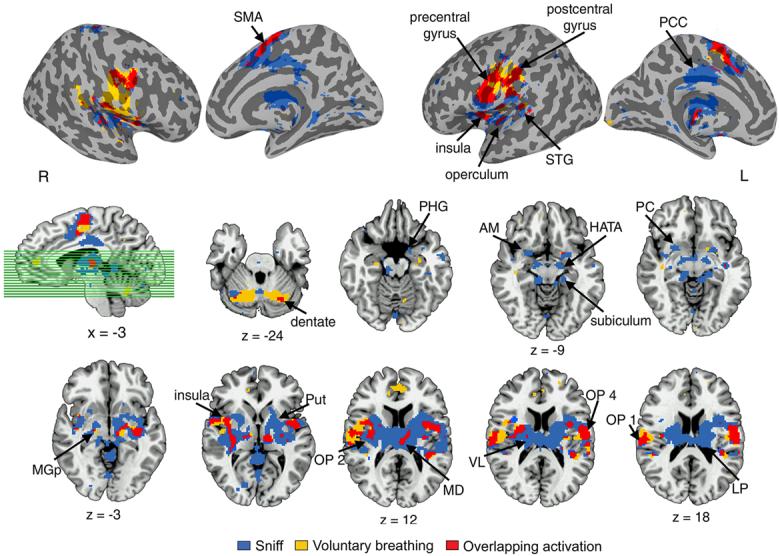
Mean cortical activation is presented on the inflated cortical surfaces; subcortical activation is shown in the series of axial brain images of the single subject template in the Talairach-Tournoux standard space (cluster size ≥ 8 contiguous voxels at p = 0.001). The sagittal image shows the location of the axial cut-planes. Plane coordinates are displayed, respectively. SMA – supplementary motor area; PCC – posterior cingulate cortex; STG – superior temporal gyrus; PHG – parahippocampal gyrus; AM – amygdala; HATA – hippocampal-amygdaloid transitional area; PC – piriform cortex; MGp – medial globus pallidus; Put – putamen; OP – operculum; VL – ventrolateral nucleus; MD – medial dorsal nucleus; LP – lateral posterior thalamus; L - left hemisphere; R - right hemisphere.
Similar to voluntary cough, voluntary sniff production recruited greater overall volume of activation (total volume of 14,326 mm3) than voluntary breathing (6,587 mm3). The ROI analysis of mean percent activation volume between voluntary sniff and breathing showed a significant task effect (F1,14 = 16.892, p = 0.001) and significant task by ROI (F12,168 = 6.398, p = 0.0005) and task by ROI by hemispheric laterality (F12,168 = 3.377, p = 0.001) interactions. During sniffing, percent activation volume was significantly greater in the premotor cortex (area 6: F1,14 = 7.478, p = 0.016), operculum (OP 2: F1,14 = 6.829, p = 0.020), ventrolateral thalamus (F1,14 = 9.623, p = 0.008), and the putamen (F1,14 = 11.295, p = 0.005) (Fig. 5b).
Comparison between cough and sniff
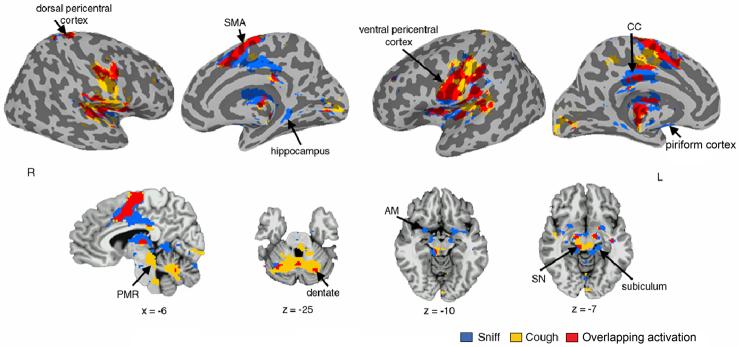
The only region exclusively active during voluntary coughing, but not during voluntary sniffing or breathing, was the region at the ponto-mesencephalic junction, extending to the brainstem. Activation during sniffing only was elicited in the piriform cortex and the hippocampus proper bilaterally (Fig. 6). Both voluntary cough and sniff resulted in equal overall volumes of activation (14,060 mm3 and 14,326 mm3, respectively). Similarly, the ROI analysis showed no significant task effects in the sensorimotor activation volume between cough and sniff productions.
Figure 6. Group mean conjoint and distinct activation during voluntary sniffing and voluntary coughing.
Activation is presented on the inflated cortical surfaces and the sagittal and axial brain images of the single subject template in the Talairach-Tournoux standard space. Plane coordinates are displayed, respectively (p ≥ 0.05, corrected). R – right hemisphere; L – left hemisphere; SMA – supplementary motor area; CC – cingulate cortex; PMR – pontomesencephalic region; SN – substantia nigra; AM – amygdala.
Discussion
Voluntary coughing and sniffing involve similar sensorimotor regions as voluntary breathing
Our results indicate that neural control underlying different voluntary respiratory behaviors integrates brain regions, extending from the neocortex to the brainstem. The common components involve the ventrolateral pericentral cortex for complex upper airway and orofacial movements, the dorsolateral cortex for respiratory movements, the SMA and cingulate cortex for higher-order motor preparation and processing, the insula/operculum for integration of sensory input with motor modalities, and the subcortical structures for processing and transfer of sensorimotor information to the higher cortical regions. Voluntary coughing and sniffing elicit substantially larger volumes of activation within the same sensorimotor regions compared with voluntary breathing. Because in our experimental setting cough and sniff were produced voluntarily without specific sensory triggers (e.g., irritation of the airways or smell presentation), increased activation response in brain motor regions during these tasks likely resulted from increased motor control demands to coordinate and modulate breathing that further recruited sensory brain regions for task perception. Although no specific sensory stimuli were presented during this study, task-specific changes in the respiratory tract (e.g., asymmetry in airflow through the nasal passages, lung volume and glottal changes) may have played an important role in modulation of different sensory input during voluntary coughing, sniffing and breathing. Consequently, these differences in the afferent input may have resulted in task-specific differences in somatosensory activation during experimental task production. In addition, somewhat different volumes of air through the nostrils during sniffing may have introduced asymmetries in brain activation in our subjects when both nostrils were exposed to air in the scanning room. A limitation of this study is that its design did not allow us to distinguish between motor activity during a respiratory behavior and sensory feedback (e.g., olfactory, proprioceptive) as a consequence of a behavior. Future neuroimaging studies, therefore, should address the effects of sensory feedback and motor performance on brain activation separately during complex respiratory tasks.
The largest region of activation during voluntary coughing, sniffing and breathing involved the ventrolateral sensorimotor cortex of the orofacial representation (Penfield and Rasmussen, 1950), extending to the insula and fronto-parietal operculum, for processing functionally linked voluntary respiratory and orofacial behaviors. These brain regions are regularly active during production of isolated orofacial tasks without respiratory movements, such as chewing, lip pursing, tongue movements (Lotze et al., 2000; Onozuka et al., 2002) as well as during orofacial movements with involvement of voluntary respiratory control, such as speaking, singing, and swallowing (Martin et al., 2004; Perry et al., 1999; Soros et al., 2006). A smaller region of activation during all voluntary respiratory tasks in this study was located in the dorsolateral sensorimotor cortex, where the respiratory muscles for breathing control are represented (Penfield and Rasmussen, 1950; Ramsay et al., 1993). Dissociation between these two foci of activation for voluntary respiratory/orofacial control within the sensorimotor cortex may be critical to the development of cough and sniff dysfunction in neurological disorders.
Voluntary coughing and sniffing involve distinct brain regions
While voluntary coughing and sniffing initiated convergent activity in the sensorimotor cortical regions, they also recruited distinct activity in the specialized subcortical structures. During voluntary coughing, a large extent of activation was observed at the midbrain-pons transition, including the parabrachial region. Similar activation was reported during voluntary suppression of breathing (McKay et al., 2003). Single-unit recording studies in the cat and monkey have shown that vocalization-correlated neurons in the parabrachial region increase their activity during expiratory or the late inspiratory phase (Farley et al., 1992; Kirzinger and Jurgens, 1991). During voluntary cough, the region at the ponto-mesencephalic junction is likely gating the facilitatory pathway between the cortical and brainstem cough centers. Together, these data may suggest involvement of this region in fine-tuning of respiratory behavior for voluntary modulation of the expiratory component for cough production.
During sniffing, the differential cortical activation involving the olfactory percept representation is consistent with the previous imaging studies (Sobel et al., 1998a; Zald and Pardo, 2000). Although no odor was introduced in this study, activation in the piriform cortex during sniffing may have recruited sensory and attentional mechanisms as automatic preparedness in odor exploration. Enhanced activation in the hippocampal region suggests its particular role in maintenance of forced inspiratory efforts during sniffing. Previously, the dentate gyrus was shown to generate rhythmic slow activity synchronized with the respiratory rhythm of sniffing, but unrelated to accompanying hippocampal olfactory beta and gamma waves (Macrides, 1975; Vanderwolf, 2001). Because of its strong olfactory and cerebellar projections (Johnson et al., 2003; Mainland and Sobel, 2006), the hippocampal region may indeed represent a link between the olfactomotor and sensorimotor regulation pathways during sniff production.
Functional significance
Cough is the most common symptom of respiratory disease, which may persist for weeks or even months. On the other hand, cough failure in neurological patients may be a life-threatening condition leading to aspiration pneumonia. Centrally acting cough suppressants or cough-inducing agents are thought to reorganize and modify the responsiveness of various components of the central cough pathway (Bolser, 1996). However, little is known about the mechanisms of action of the most of these drugs. Understanding the functional organization of the central cough system in humans may allow targeting specific brain regions with pharmacological agents to modulation cough response in patients.
At present, sniffing for olfactory percept is thought to be controlled subcortically based on the observations of latency for olfactory cortical evoked potentials (Johnson et al., 2003). Our study demonstrates that sniff production involves cortical and subcortical control of both respiratory and olfactory subcomponents. A new view on functional localization of central sniff control within the respiratory and olfactory percept systems may shed light on the mechanisms of sniff and smell deficits in patients with stroke and neurodegenerative diseases (Abele et al., 2003; Connelly et al., 2003; Mainland et al., 2005; Sobel et al., 2001). If impaired olfaction results from sniff dysfunction, an inability to perform sniffing is likely associated with either suppression of the cortical sensorimotor sniff pathway or disintegration of the olfactomotor and sensorimotor sniff control systems.
Supplementary Material
Acknowledgements
We thank Richard Reynolds and Gang Chen for assistance with data analysis. This work was supported by the Intramural Program of the National Institute of Neurological Disorders and Stroke, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abele M, Riet A, Hummel T, Klockgether T, Wullner U. Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J Neurol. 2003;250:1453–1455. doi: 10.1007/s00415-003-0248-4. [DOI] [PubMed] [Google Scholar]
- Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke: an interhospital comparison. Stroke. 1999;30:1203–1207. doi: 10.1161/01.str.30.6.1203. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC. Mechanisms of action of central and peripheral antitussive drugs. Pulm Pharmacol. 1996;9:357–364. doi: 10.1006/pulp.1996.0047. [DOI] [PubMed] [Google Scholar]
- Chung KF, Chang AB. Therapy for cough: active agents. Pulm Pharmacol Ther. 2002;15:335–338. doi: 10.1006/pupt.2002.0342. [DOI] [PubMed] [Google Scholar]
- Connelly T, Farmer JM, Lynch DR, Doty RL. Olfactory dysfunction in degenerative ataxias. J Neurol Neurosurg Psychiatry. 2003;74:1435–1437. doi: 10.1136/jnnp.74.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006a;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006b;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Farley GR, Barlow SM, Netsell R. Factors influencing neural activity in parabrachial regions during cat vocalizations. Exp Brain Res. 1992;89:341–351. doi: 10.1007/BF00228250. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson's disease. Am J Respir Crit Care Med. 1998;158:458–464. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Springer; Wien: 2003. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Hadjikoutis S, Eccles R, Wiles CM. Coughing and choking in motor neuron disease. J Neurol Neurosurg Psychiatry. 2000;68:601–604. doi: 10.1136/jnnp.68.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings HA, Eccles R, Smith AP, Jawad MS. Voluntary cough suppression as an indication of symptom severity in upper respiratory tract infections. Eur Respir J. 1993a;6:1449–1454. [PubMed] [Google Scholar]
- Hutchings HA, Morris S, Eccles R, Jawad MS. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993b;87:379–382. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- Jamal K, McMahon S, Edgell G, Fleetham JA. Cough and arousal responses to inhaled citric acid in sleeping humans. Am Rev Respir Dis. 1983;127:237. [Google Scholar]
- Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90:1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- Karlsson JA, Sant'Ambrogio G, Widdicombe J. Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol. 1988;65:1007–1023. doi: 10.1152/jappl.1988.65.3.1007. [DOI] [PubMed] [Google Scholar]
- Kirzinger A, Jurgens U. Vocalization-correlated single-unit activity in the brain stem of the squirrel monkey. Exp Brain Res. 1991;84:545–560. doi: 10.1007/BF00230967. [DOI] [PubMed] [Google Scholar]
- Lee PC, Cotterill-Jones C, Eccles R. Voluntary control of cough. Pulm Pharmacol Ther. 2002;15:317–320. doi: 10.1006/pupt.2002.0365. [DOI] [PubMed] [Google Scholar]
- Lee PC, Jawad MS, Hull JD, West WH, Shaw K, Eccles R. The antitussive effect of placebo treatment on cough associated with acute upper respiratory infection. Psychosom Med. 2005;67:314–317. doi: 10.1097/01.psy.0000155667.59662.92. [DOI] [PubMed] [Google Scholar]
- Lotze M, Seggewies G, Erb M, Grodd W, Birbaumer N. The representation of articulation in the primary sensorimotor cortex. Neuroreport. 2000;11:2985–2989. doi: 10.1097/00001756-200009110-00032. [DOI] [PubMed] [Google Scholar]
- Macrides F. Temporal relationships between hippocampal slow waves and exploratory sniffing in hamsters. Behav Biol. 1975;14:295–308. doi: 10.1016/s0091-6773(75)90419-8. [DOI] [PubMed] [Google Scholar]
- Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses. 2006;31:181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N. Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci. 2005;25:6362–6371. doi: 10.1523/JNEUROSCI.0920-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- Nishino T, Hiraga K, Mizuguchi T, Honda Y. Respiratory reflex responses to stimulation of tracheal mucosa in enflurane-anesthetized humans. J Appl Physiol. 1988;65:1069–1074. doi: 10.1152/jappl.1988.65.3.1069. [DOI] [PubMed] [Google Scholar]
- Nishino T, Isono S, Tanaka A, Ishikawa T. Laryngeal inputs in defensive airway reflexes in humans. Pulm Pharmacol Ther. 2004;17:377–381. doi: 10.1016/j.pupt.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, Saito S. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2002;81:743–746. doi: 10.1177/0810743. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The cerebral cortex of man: A clinical study of localization of function. Macmillan; New York: 1950. [Google Scholar]
- Perry DW, Zatorre RJ, Petrides M, Alivisatos B, Meyer E, Evans AC. Localization of cerebral activity during simple singing. Neuroreport. 1999;10:3979–3984. doi: 10.1097/00001756-199912160-00046. [DOI] [PubMed] [Google Scholar]
- Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, Frackowiak RS, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol. 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998a;392:282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998b;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Thomason ME, Stappen I, Tanner CM, Tetrud JW, Bower JM, Sullivan EV, Gabrieli JD. An impairment in sniffing contributes to the olfactory impairment in Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:4154–4159. doi: 10.1073/pnas.071061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT. Clustered functional MRI of overt speech production. Neuroimage. 2006;32:376–387. doi: 10.1016/j.neuroimage.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Stephens RE, Addington WR, Widdicombe JG. Effect of acute unilateral middle cerebral artery infarcts on voluntary cough and the laryngeal cough reflex. Am J Phys Med Rehabil. 2003;82:379–383. doi: 10.1097/01.PHM.0000064730.54787.F5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planer stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tomori Z, Benacka R, Donic V, Tkacova R. Reversal of apnoea by aspiration reflex in anaesthetized cats. Eur Respir J. 1991;4:1117–1125. [PubMed] [Google Scholar]
- Vanderwolf CH. The hippocampus as an olfacto-motor mechanism: were the classical anatomists right after all? Behav Brain Res. 2001;127:25–47. doi: 10.1016/s0166-4328(01)00354-0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Eccles R, Fontana G. Supramedullary influences on cough. Respir Physiol Neurobiol. 2006;152:320–328. doi: 10.1016/j.resp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Fontana G. Cough: what's in a name? Eur Respir J. 2006;28:10–15. doi: 10.1183/09031936.06.00096905. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Singh V. Physiological and pathophysiological down-regulation of cough. Respir Physiol Neurobiol. 2006;150:105–117. doi: 10.1016/j.resp.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT, Zhang Z, Baekey DM, Shannon R. Cerebellar modulation of cough motor pattern in cats. J Appl Physiol. 1997;83:391–397. doi: 10.1152/jappl.1997.83.2.391. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36:165–181. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.