Abstract
2B4 (CD244), a member of the CD2 subset of the immunoglobulin superfamily, is important for stimulating human natural killer (NK) cell cytotoxicity and cytokine production. It is expressed on all NK cells, a subpopulation of T cells, monocytes, basophils and eosinophils. 2B4 interaction with its ligand CD48 regulates NK, T and B lymphocyte functions and thus plays a central role in various immune responses. Previous study indicated a role for AP-1 and Ets in the transcription of the 2B4 gene. In this study we report that stimulation of NK cells through surface 2B4 down-regulates its own expression due to a reduction in the promoter activity at the Ets element. The down-regulation of 2B4 could be a mechanism to attenuate the co-stimulatory signal from 2B4–CD48 interactions.
Keywords: Natural killer (NK) cell, 2B4 promoter, Transcription regulation, Ets element
Natural killer cells are part of the first line of defense against pathogens and cancer cells [1]. They express several receptors that recognize ligands on potential target cells. The natural cytotoxicity and cytokine secretion is mediated through a delicate balance of signals received through activating and inhibitory receptors expressed by the NK cells [2]. A variety of different surface receptors can induce NK cell activation.
2B4 (CD244) was initially discovered as an activating receptor on murine NK cells and a subset of T cells that mediate non-MHC restricted cytotoxicity and later the human homologue of mouse 2B4 was cloned [3,4]. However, recent studies indicate that 2B4 acts as an inhibitory receptor on murine NK cells [5,6]. Molecular cloning of human 2B4 by us and others revealed that 2B4 plays a broader role in the immune system [4,7]. Unlike in murine NK cells, 2B4 acts predominantly as an activation receptor on human NK cells [8]. In humans, 2B4 is expressed on all NK cells, γδ T cells, monocytes, basophils, and on a subset of CD8+ T cells. Ligation of surface 2B4 with a monoclonal antibody or its ligand CD48 stimulate NK-cell cytotoxicity, production of stimulatory cytokines such as interferon-γ (IFN-γ), and granule exocytosis [7,9]. In addition, T cells have been shown to acquire 2B4 expression under certain activating conditions. For instance, murine CD8+ T cells acquire 2B4 expression upon cytokine stimulation in vitro and during viral infection in vivo [10]. 2B4 is also found on a large proportion of effector/memory CD4+ T cells in CMV-infected individuals [11]. 2B4 can effectively costimulate the signals of other activating NK cell receptors [12] and can enhance the cytotoxic activity of antigen-specific T cells [13]. 2B4 can also function as an activating NK cell ligand. 2B4 expression on target cells can stimulate NK cell cytotoxicity and IFN-γ production through the engagement of CD48 on NK cells [14]. Through sites of tyrosine phosphorylation in its cytoplasmic domain, 2B4 associates with the signaling lymphocyte activation molecule (SLAM)-associated protein (SAP; also called SH2D1A), an intracellular adaptor molecule composed of an Src homology 2 (SH2) domain and a short C-terminal tail [15,16]. SAP is expressed in NK cells, T cells, NKT cells, and some B cells and is altered in X-linked lymphoproliferative disease in humans. Earlier we cloned a 1.1 kb 5′ regulatory region of the 2B4 gene and mapped regulatory regions within this promoter fragment [17]. AP1 was shown to play an important role in 2B4 gene transcription. Another study identified an activating Ets element in the 2B4 promoter 945 bp upstream of the transcription start site, which is AP1 dependent [18].
Previously we have shown that YT cells, a human NK cell line, have a high expression of h2B4 and demonstrated increased cytolytic activity upon stimulation with mAb C1.7, which recognizes 2B4. Stimulation of YT cells by mAb C1.7 increases production of IFN-γ, upregulates the expression of matrix metalloproteinases (MMP-2) and modulates the expression of h2B4 [19]. But prolonged stimulation of human PBMC stimulated with mAb C1.7 led to a drastic decrease in mRNA levels of h2B4 after 4 h. In this study, we examined the activity of the AP1 and Ets element in YT cells upon 2B4 stimulation to find out whether down-regulation of the 2B4 gene is mediated through their activity.
Materials and methods
Cell culture and preparation of nuclear extract.
YT (human NK cell line) cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Hyclone Lab, Logan, UT), 10 mM Hepes, 10 mM non-essential amino acids, 100 U/ml penicillin, 100 U/ml streptomycin, and 1 mM sodium pyruvate (Life Technologies, Gaithersburg, MD). Cells were maintained at 37 °C in 5% CO2. YT nuclear extract was prepared as described previously [17]. Briefly, 50 million YT cells were lysed in hypotonic Buffer A (10 mM Hepes, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, and 50 mM PMSF) to release the nuclei. Nuclear protein extraction buffer C (20 mM Hepes, pH 7.5, 1.5 mM MgCl2, 600 mM NaCl, 0.2 mM EDTA, 20% glycerol, 1 mM DTT, and 50 mM PMSF) was then used to extract the proteins from the nuclei. Protein extract was aliquoted and stored at −70 °C until use.
Dual luciferase reporter assay.
Full length, wild type human 2B4 (WT) promoter construct in pGL2-Basic vector (Promega, Madison, WI) was made as described previously [17]. All mutant constructs were made from this construct by in vitro mutagenesis. AP1 and EtsM mutants were constructed as described previously [18]. One million YT cells were transiently transfected with 4 μg of reporter construct and 0.4 μg of Renilla luciferase reporter plasmid using Fugene transfection reagent (Roche, Indianapolis, IN). Firefly and Renilla luciferase assays were performed using the Dual Luciferase Reporter Assay System (Promega, Madison, WI) as per manufacturer’s instructions, 48 h later.
Reverse transcription PCR.
YT cells were stimulated with mAb C1.7 for 30 min, 1, 2, and 4 h at 37 °C and total RNA was isolated with the RNAstat 60 reagent according to the manufacturer’s protocol (Teltest Inc., Friendswood, TX) and first strand cDNA was synthesized from 2 μg of total RNA using Omniscript RT (Qiagen) reverse transcriptase and random primers in a volume of 20 μl. PCR was performed using 100 ng of cDNA under standard conditions for 35 cycles. PCR was performed using sense primer (5′-GCTCTTTGCCTTCCAATACTTCC-3′) and antisense primer (5′-CCTCTTCCTGGATCCAGACTTCC-3′). Oligonucleotides were custom synthesized by Integrated DNA Technologies (Coraville, IA).
Electrophoretic mobility shift assays (EMSA).
A 25 bp oligonucleotide (5′-GCAGATCCAGAGCTGCCTGGCCTTC-3′) corresponding to nucleotides −955 to −931 (spanning the −945 hypersensitive site) and nuclear extract from YT cells stimulated with C1.7 were used in the EMSA. Labeled probes were incubated with YT cell nuclear extract on ice for 30 min in a 20 μl reaction containing 10 mM Tris (pH 7.5), 1 mM EDTA, 1 mM DTT, 5% glycerol, 4 mM MgCl2, 2 μg poly(dI–dC). Samples were electrophoresed on a 4% non-denaturing polyacrylamide gel at 200 V for 60−70 min. Gels were then dried and exposed to X-ray film.
Results and discussion
2B4 stimulation down-regulates its own expression
To determine the role of cis-acting elements in the 2B4 promoter, we performed Luciferase reporter assays in YT cells using a full length wild type promoter and two mutants (Ets and AP-1) as described previously [18]. The activity of the wild type construct without C1.7 stimulation was set at 100% and activity of other constructs are relative to this activity. The reporter assay shows that 2B4 promoter activity decreases to about 60% after mAb C1.7 stimulation, indicating that 2B4 down-regulates its own expression. To determine which element contributes to this down-regulation, Ets and AP1 mutant constructs were used in the reporter assay. When the Ets element is mutated, promoter activity decreases to about 60%, close to the activity after C1.7 stimulation. In this case, C1.7 does not reduce promoter activity any further, indicating that the down-regulation from C1.7 was mediated through the Ets element (Fig. 1). When the AP1 element is mutated, promoter activity drops to 30% and C1.7 is not able to down-regulate it any further. We have previously shown that the Ets element is AP1 dependent. Hence, in the AP1 mutant construct, although the Ets element is intact, it is non-functional and C1.7 cannot down-regulate promoter activity.
Fig. 1.
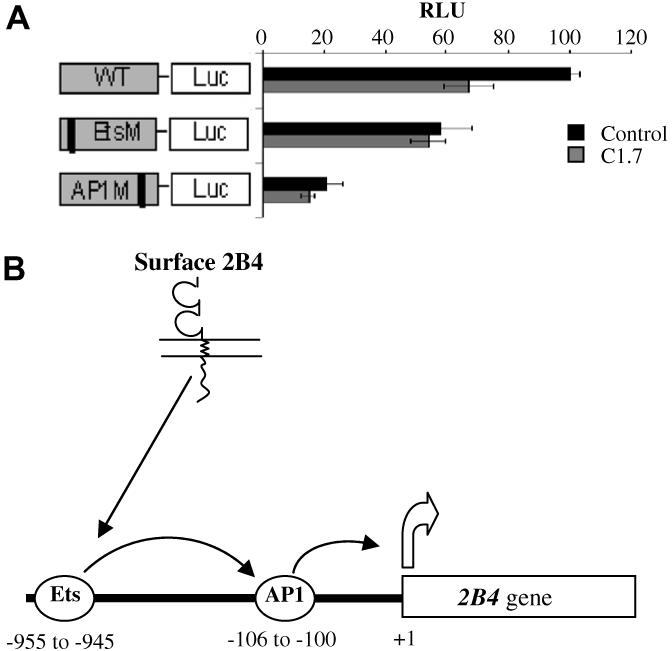
2B4 stimulation down-regulates its own expression (A) Dual luciferase assay after mAb C1.7 stimulation. Stimulation of 2B4 by C1.7 antibody decreases promoter activity. YT cells were incubated with 200 ng/ml of C1.7, 4 h prior to transfection. Constructs were then transfected along with a pRL3-CMV control vector and firefly and renilla luciferase activity was determined 40 h later. RLU, relative light units. The activity of the wild type construct without C1.7 stimulation is set at 100% and activity of other constructs are relative to this activity. Schematics of the constructs are shown to the left of the bar graphs. WT, wild type construct; EtsM, construct with mutation at Ets site; AP1M, AP1 site mutant. Error bars indicate standard deviation (each reaction was run in triplicate). (B) Schematic of 2B4 gene down-regulation upon signaling through surface 2B4. The Ets transcription factor binds at −955 to −945 bp of the 2B4 gene promoter and up-regulates transcription in an AP1 dependent manner. Reduction in the Ets binding may lead to reduced expression of 2B4 gene.
In order to determine the down-regulation of 2B4 at the RNA level, we analyzed mRNA levels of h2B4 in YT cells stimulated with mAb C1.7. RT-PCR revealed a decrease in mRNA level of h2B4 after 4 h (Fig. 2). These results corroborates with our previous findings in human NK cells stimulated with C1.7 revealing a drastic decrease in mRNA levels of h2B4 after 4 h [19].
Fig. 2.
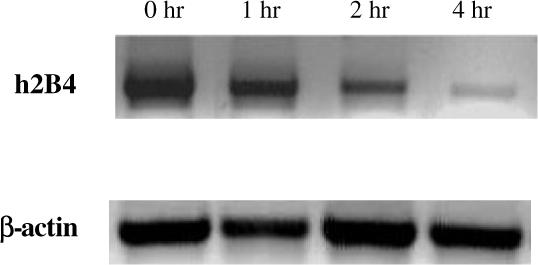
h2B4 down-regulates its own RNA expression. YT cells (1 × 106 cells/ml) were incubated in the presence of mAb C1.7 (200 ng/ml) for various time intervals. Total RNA was extracted and subjected to RT-PCR with primers amplifying sequences of the h2B4 gene and the β-actin gene. PCR products were run on a 1.2% agarose gel with ethidium bromide.
Down-regulation of h2B4 is due to reduction in Ets activity
In order to determine whether C1.7 stimulation alters binding of nuclear factor to the Ets element, we performed electrophoretic mobility shift assays. As seen in Fig. 3, incubation of the labeled oligonucleotide with YT nuclear extract produced two DNA-protein complexes. Competition assay with cold probe revealed that the higher molecular weight DNA–protein complex is specific (Fig. 3, shown by the arrow) while the lower complex is non-specific. Results show a progressive decline in the binding of the Ets element in the 2B4 promoter to the YT nuclear protein over a 4 h period (Fig. 3) and remained unchanged even after 6 h (data not shown). These results agree with the reporter assays and indicate that 2B4 down-regulates its own expression upon stimulation with 2B4. This also suggests that the down-regulation after 2B4 stimulation is due to a reduction in activity at the Ets element.
Fig. 3.
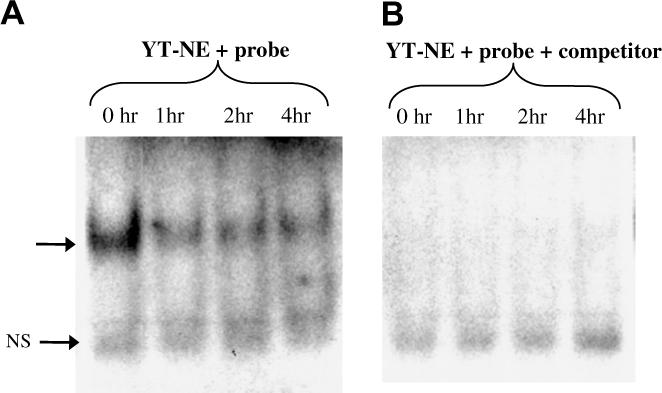
Reduced Ets-1 binding revealed by EMSA. (A) EMSA was performed with the radioactive probe and nuclear extract from YT cells (YT-NE) stimulated with C1.7 for the indicated time periods. (B) Competition assay was performed with 100 M excess of unlabeled probe. Samples were electrophoresed on a 4% non-denaturing polyacrylamide gel at 200 V for 60−70 min. Arrow indicates the position of the DNA- protein complex. NS indicates non-specific binding.
The Ets group of transcription factors play an important role in cell proliferation, differentiation, apoptosis, migration, and cell-extracellular matrix interactions; processes critical for immune cell functioning [20]. In addition, Ets-1 is necessary for the development and /or survival of the NK cell lineage in mice as indicated by the significantly reduced numbers of NK cells from Ets-1 deficient mice and reduced NK cell cytolytic activity against tumor targets in vitro [21]. Recently, it was shown that Ets-1 has multiple Ca2+-dependent phosphorylation sites for graded DNA binding affinity [22]. Interestingly, Ets-1 is known to be phosphorylated by Erk1/2 and glycogen synthase kinase 3 (GSK-3), which can be activated after 2B4 engagement [20,23,24]. Also, Ji et al. reported that Ets-1 can be modified in its two lysine residues by sumoylation, resulting in repression of transcriptional activity by Ets-1 [25]. It might be possible that antibody-mediated stimulation of 2B4 send a signal to modify Ets factors and down-regulate its own expression. Because 2B4 expression is up-regulated upon certain viral infections, Ets might play a more significant role under such conditions [26].
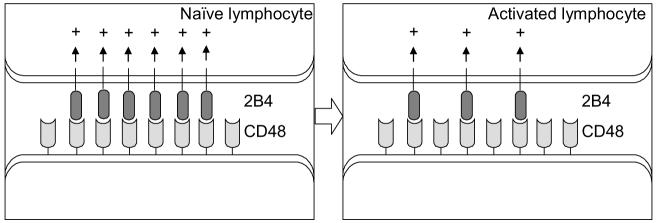
A recent study has shown the down modulation of 2B4 receptor from the surface of stimulated YTS cells due to internalization of the receptor resulting in reduced 2B4-mediated NK cell activation and cytotoxicity [27]. Therefore, stimulation of NK cells leads to the down- regulation of 2B4 at the protein and mRNA level. This raises the question—why does 2B4 down-regulate its own expression? This could be answered in light of the newly described co-stimulatory function of 2B4 on lymphocytes. Co-stimulatory molecules provide the necessary accessory signal required for the antigen-specific clonal expansion of lymphocytes. CD28 is the best-studied co-stimulatory molecule on T cells. It binds to B7.1 or B7.2 on antigen presenting cells (APCs) and this engagement in the proper context provides the co-stimulatory signal [28]. Studies indicate that 2B4 expressed on activated CD8+T cells may act as a costimulatory ligand for CD48 on neighboring T cells, enhancing proliferation and other effector functions of the latter [29]. Upon activation, the T cells express another receptor, CTLA-4, that also binds to B7 molecules on APCs. However, it delivers an inhibitory signal, limiting the proliferative response of the activated T cells. Mice lacking CTLA-4 have a fatal disorder characterized by uncontrolled proliferation of lymphocytes [30]. This demonstrates the importance of attenuating the co-stimulatory signal after lymphocytes have been activated. A recent study shows that homotypic NK cell interaction, through 2B4–CD48, is required for IL-2 driven expansion and activation of NK cells [31]. As NK cells expand one would expect even more 2B4–CD48 stimulation through the neighboring NK cells, potentially leading to a positive feedback loop of NK cell expansion and stimulation through 2B4–CD48. The down-regulation of 2B4 could be a mechanism to attenuate the stimulatory signal from 2B4–CD48 interactions. Thus, 2B4–CD48 interactions among neighboring lymphocytes could help boost the initial immune response, which would then be limited by down-regulation of 2B4 on lymphocytes (Fig. 4).
Fig. 4.
A model for controlling immune response arising through 2B4–CD48 interactions. 2B4–CD48 interactions on neighboring lymphocytes provide a co-stimulatory signal. After the initial stimulation 2B4 expression is down-regulated, possibly, to attenuate this co-stimulatory signal.
In conclusion, we have shown that stimulation of YT cells through surface 2B4 leads to a down-regulation of 2B4 expression due to a reduction in the promoter activity at the Ets element. The down-regulation of 2B4 may be an important factor in controlling lymphocyte activation during immune responses.
Acknowledgment
This work was supported by the NIH Grant CA85753 to P.A.M.
References
- 1.Lanier LL. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Activating and inhibitory NK cell receptors. Adv. Exp. Med. Biol. 1998;452:13–18. doi: 10.1007/978-1-4615-5355-7_2. [DOI] [PubMed] [Google Scholar]
- 3.Mathew PA, Garni-Wagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J. Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- 4.Boles KS, Nakajima H, Colonna M, Chuang SS, Stepp SE, Bennett M, Kumar V, Mathew PA. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27–34. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya SV, Stepp SE, McNerney ME, Lee JK, Bennett M, Lee KM, Stewart CL, Kumar V, Mathew PA. Targeted disruption of the 2B4 gene in mice reveals an in vivo role of 2B4 (CD244) in the rejection of B16 melanoma cells. J. Immunol. 2005;174:800–807. doi: 10.4049/jimmunol.174.2.800. [DOI] [PubMed] [Google Scholar]
- 6.Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur. J. Immunol. 1999;29:1676–1683. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Mathew SO, Kumaresan PR, Lee JK, Huynh VT, Mathew PA. Mutational analysis of the human 2B4 (CD244)/CD48 interaction: Lys68 and Glu70 in the V domain of 2B4 are critical for CD48 binding and functional activation of NK cells. J. Immunol. 2005;175:1005–1013. doi: 10.4049/jimmunol.175.2.1005. [DOI] [PubMed] [Google Scholar]
- 9.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J. Exp. Med. 1993;178:1397–1406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kambayashi T, Assarsson E, Chambers BJ, Ljunggren HG. Cutting edge: Regulation of CD8(+) T cell proliferation by 2B4/CD48 interactions. J. Immunol. 2001;167:6706–6710. doi: 10.4049/jimmunol.167.12.6706. [DOI] [PubMed] [Google Scholar]
- 11.Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, Mackay CR, Cooper DA, Saksena NK, Kelleher AD. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103:2238–2247. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]
- 12.Sivori S, Parolini S, Falco M, Marcenaro E, Biassoni R, Bottino C, Moretta L, Moretta A. 2B4 functions as a co-receptor in human NK cell activation. Eur. J. Immunol. 2000;30:787–793. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Lee KM, Bhawan S, Majima T, Wei H, Nishimura MI, Yagita H, Kumar V. Cutting Edge: The NK Cell Receptor 2B4 Augments Antigen-Specific T Cell Cytotoxicity Through CD48 Ligation on Neighboring T Cells. J. Immunol. 2003;170:4881–4885. doi: 10.4049/jimmunol.170.10.4881. [DOI] [PubMed] [Google Scholar]
- 14.Messmer B, Eissmann P, Stark S, Watzl C. CD48 stimulation by 2B4 (CD244)-expressing targets activates human NK cells. J. Immunol. 2006;176:4646–4650. doi: 10.4049/jimmunol.176.8.4646. [DOI] [PubMed] [Google Scholar]
- 15.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 2003;3:813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- 16.Roncagalli R, Taylor JE, Zhang S, Shi X, Chen R, Cruz-Munoz ME, Yin L, Latour S, Veillette A. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat. Immunol. 2005;6:1002–1010. doi: 10.1038/ni1242. [DOI] [PubMed] [Google Scholar]
- 17.Chuang SS, Pham HK, Kumaresan PR, Mathew PA. A prominent role for activator protein-1 in the transcription of the human 2B4 (CD244) gene in natural killer cells. J. Immunol. 2001;166:6188–6195. doi: 10.4049/jimmunol.166.10.6188. [DOI] [PubMed] [Google Scholar]
- 18.Vaidya SV, Mathew PA. An Ets element regulates the transcription of the human 2B4 gene in natural killer cells. Biochim. Biophys. Acta. 2005;1728:181–185. doi: 10.1016/j.bbaexp.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Chuang SS, Kim MH, Johnson LA, Albertsson P, Kitson RP, Nannmark U, Goldfarb RH, Mathew PA. 2B4 stimulation of YT cells induces natural killer cell cytolytic function and invasiveness. Immunology. 2000;100:378–383. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tootle TL, Rebay I. Post-translational modifications influence transcription factor activity: a view from the ETS superfamily. Bioessays. 2005;27:285–298. doi: 10.1002/bies.20198. [DOI] [PubMed] [Google Scholar]
- 21.Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL, Leiden JM. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–563. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- 22.Pufall MA, Lee GM, Nelson ML, Kang HS, Velyvis A, Kay LE, McIntosh LP, Graves BJ. Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science. 2005;309:142–145. doi: 10.1126/science.1111915. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Holm M, Xie XQ, Wolf-Watz M, Grundstrom T. AML1/Runx1 recruits calcineurin to regulate granulocyte macrophage colony-stimulating factor by Ets1 activation. J. Biol. Chem. 2004;279:29398–29408. doi: 10.1074/jbc.M403173200. [DOI] [PubMed] [Google Scholar]
- 24.Aoukaty A, Tan R. Role for glycogen synthase kinase-3 in NK cell cytotoxicity and X-linked lymphoproliferative disease. J. Immunol. 2005;174:4551–4558. doi: 10.4049/jimmunol.174.8.4551. [DOI] [PubMed] [Google Scholar]
- 25.Ji Z, Degerny C, Vintonenko N, Deheuninck J, Foveau B, Leroy C, Coll J, Tulasne D, Baert JL, Fafeur V. Regulation of the Ets-1 transcription factor by sumoylation and ubiquitinylation. Oncogene. 2007;26:395–406. doi: 10.1038/sj.onc.1209789. [DOI] [PubMed] [Google Scholar]
- 26.Ostrowski SR, Ullum H, Pedersen BK, Gerstoft J, Katzenstein TL. 2B4 expression on natural killer cells increases in HIV-1 infected patients followed prospectively during highly active antiretroviral therapy. Clin. Exp. Immunol. 2005;141:526–533. doi: 10.1111/j.1365-2249.2005.02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandusky MM, Messmer B, Watzl C. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur. J. Immunol. 2006;36:3268–3276. doi: 10.1002/eji.200636146. [DOI] [PubMed] [Google Scholar]
- 28.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J. Exp. Med. 1993;177:845–850. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assarsson E, Kambayashi T, Persson CM, Ljunggren HG, Chambers BJ. 2B4 co-stimulation: NK cells and their control of adaptive immune responses. Mol. Immunol. 2005;42:419–423. doi: 10.1016/j.molimm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee KM, Forman JP, McNerney ME, Stepp S, Kuppireddi S, Guzior D, Latchman YE, Sayegh MH, Yagita H, Park CK, Oh SB, Wulfing C, Schatzle J, Mathew PA, Sharpe AH, Kumar V. Requirement of homotypic NK-cell interactions through 2B4(CD244)/CD48 in the generation of NK effector functions. Blood. 2006;107:3181–3188. doi: 10.1182/blood-2005-01-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]