Abstract
Macrophage migration inhibitory factor (MIF) is a multi-functional cytokine that is considered a pro-inflammatory cytokine. However, our studies show that MIF, when produced in super-physiological levels by a murine neuroblastoma cell line (Neuro-2a) exceeding those normally seen during an immune response, inhibits cytokine-, CD3-, and allo-induced T-cell activation. MIF is also able to inhibit T cells that have already received an activation signal. The T-cell inhibitory effects of culture supernatants from neuroblastoma cells were reversed when the cells were transfected with dicer-generated si-RNA to MIF. When T cells were activated in vitro by co-culture with interleukin (IL)-2 and IL-15 and analyzed for cytokine production in the presence or absence of MIF-containing culture supernatant, inhibition of T-cell proliferation and induced cell death were observed even as the treated T cells produced high levels of interferon-gamma (IFN-γ). The inhibitory effects of MIF were partially reversed when lymphocytes from IFN-γ knockout mice were tested. We propose that the high levels of MIF produced by neuroblastoma cause activation induced T-cell death through an IFN-γ pathway and may eliminate activated T cells from the tumor microenvironment and thus contribute to escape from immune surveillance.
Keywords: Migration inhibitory factor, Tumor immunity, T cells, Cytokines, Cell activation
1. Introduction
Macrophage migration inhibitory factor (MIF) was first described as an activator of macrophage function that inhibits random macrophage migration. MIF is a 12.5 kDa protein secreted by macrophages, T cells, and other tissues, notably the anterior pituitary, that induces both gram-negative septic shock and delayed-type hypersensitivity reactions [1–4]. MIF is unique in that the protein sequence and structural studies have not identified any cytokine family within which MIF can be placed. MIF is the only cytokine that is upregulated by glucocorticoids, and thus MIF plays a role in regulating host global responses to infection as glucocorticoid hormones are released from the hypothalamus-pituitary-adrenal-axis [3,5]. Although a naturally occurring substrate has not been identified, MIF also has enzymatic properties and exhibits tautomerase and redox activity. This structural aspect of MIF is somewhat reminiscent of cyclophilin, which functions intracellularly as a peptidy-prolyl cis-trans isomerase; and can be secreted in response to proinflammatory stimuli [6,7]. Studies have shown that MIF can override glucocorticoid inhibition of T-cell activation [8]. Furthermore, studies with a neutralizing anti-MIF antibody have shown that depletion of MIF from activated T-cell cultures inhibits T-cell proliferation in vivo and can inhibit the development of DTH lesions [8,9].
Malignant tissues are able to blunt or alter anti-tumor immune responses by the production of soluble factors that act directly on lymphocytes [10,11]. In our studies of the immune response to neuroblastoma, we found that both murine neuroblastoma cell lines and low passage clinical tumor isolates from either surgical resection of primary disease or bone marrow metastases produce MIF [12]. The list of other cytokines produced by neuroblastoma that could potentially influence the anti-tumor immune response also includes tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, and IL-15 [13–16]. The prevalence of each of these factors remains to be established, as in our limited analysis of clinical isolates only 1 in 5 produced IL-6, while 4 of 5 produced MIF. MIF has pleiotropic effects on both tumor cells and lymphocytes. MIF derived from neuroblastoma displays the classical functional activity of decreasing macrophage migration from glass capillary tubes, while also increasing transwell migration of tumor [12]. Studies with either tumor-derived, a parasite-encoded homolog, or recombinantly expressed MIF demonstrated that MIF not only inhibits macrophage random migration, but that it also plays a role in directional movement of tumor cells and macrophages [12,17,18].
The production of MIF by tumors appears contrary to its reported immuno-stimulatory activity. However, MIF plays a broad role in growth regulation in a number of systems. MIF is able to antagonize p53-mediated gene activation and apoptosis, and benzo[a]pyrene-induced fibrosarcomas in MIF knockout mice were smaller in size and had a lower mitotic index [19]. The MIF receptor has not been identified and is still under active investigation. CD74, the cell surface form of major histocompatibility class II-associated invariant chain, has been demonstrated to bind MIF with high affinity and is required for MIF-mediated extracellular signal-regulated kinase (ERK)-1/2 phosphorylation and PGE2 production in defined systems [20]. MIF has also been shown to have intracellular function in the regulation of cell growth via Jab1, a co-activator of AP-1 transcription [21].
With regard to the immune system, MIF is considered a broad-spectrum proinflammatory cytokine. MIF has been shown to stimulate TNF-α synthesis, is detected in DTH-reactive cells, and has been found to stimulate cognate T-cell activation [3,22]. T cells activated by specific antigen, mitogen, or anti-CD3 antibody increase MIF mRNA expression and secretion of protein. Anti-MIF antibodies inhibit T-cell proliferation and B cell antibody production in vivo [8]. However, some studies have demonstrated negative effects of MIF on T-cell immunity. EG7 tumor-bearing mice treated with anti-MIF antibody show an increase in CD4 and CD8 T-cell accumulation at the tumor site and an increase in T-cell accumulation of adoptively transferred T cells [23]. Thus, decreasing MIF augmented the immune response to EG7. In studies described here, we sought to determine if tumor-encoded MIF has the potential to impact anti-tumor immunity. We found that high levels of tumor-derived MIF inhibited T-cell activation by preventing cell cycle progression and augmenting apoptosis through a specific signaling pathway that involved T lymphocyte-secreted interferon-gamma (IFN-γ). We speculate that the high levels of MIF produced by tumor cells results in activation-induced cell death of tumor-infiltrating T lymphocytes, thereby suppressing anti-tumor immunity.
2. Materials and methods
2.1. Mice, tumor cells and reagents
A/J, C57BL/6 (termed B6) mice and IFN-γ knockout mice (B6.129S7-Ifngtm1Ts/J) were purchased from The Jackson Laboratory (Bar Harbor, MA) and used at 4−6 weeks of age. Mice were housed in the Biomedical Research Center at the Medical College of Wisconsin according to institutional guidelines. AGN2a is an aggressive subclone of the mouse Neuro-2a neuroblastoma cell line generated by serial in vivo passage [24]. Permanent cell lines transfected to express CD80 and CD137L (AGN2a/CD80−137L) were generated and cultured as described previously [25]. The human osteosarcoma cell line U2OS, was kindly provided by Dr. Kent Wilcox, Medical College of Wisconsin, Milwaukee. Rabbit polyclonal antibody to rat MIF (cross-reactive with mouse MIF) and AP-conjugated goat anti-rabbit IgG were obtained from Abcam, Inc. (Cambridge, MA). Annexin V-FITC and mouse Th1/Th2 cytometric bead array detection kits were from BD Biosciences Pharmingen (San Diego, CA). Recombinant mouse IFN-γ was purchased from BD-Pharmingen.
2.2. d-siRNA generation
In order to knockdown endogenous MIF expression in AGN2a cell lines, MIF-specific diced-small interfering RNA (d-siRNA) was generated using the Dicer siRNA Generation Kit from Gene Therapy System (GTS, San Diego, CA). A full length AGN2a cDNA-derived molecular clone of MIF was generated in our lab using the following primers that include both T7 and MIF sequence: fwd. 5′-GCGTAATACGACTCACTATAGGGAGAGCCACCATGCCTATGTTCATC, rev. 5′-GCGTAATACGACTCACTATAGGGAGAGACTCAAGCCAAGGTGGAAC, PCR amplified material cloned into the pCR2.1 Topo vector (Invitrogen) and then subcloned into pUC18. MIF sequences were PCR amplified by Taq polymerase, double-stranded RNA (dsRNA) transcribed from amplification products using the TurboScript T7 transcription reaction, d-siRNA generated by enzymatic digestion with dicer, and the digested material column-purified to remove salt and undigested dsRNA according to the manufacturer's protocol. Primers for GFP and GFP plasmid were supplied by GTS and used for generation of control d-siRNA. The concentration of d-siRNA was determined by the following formula: A260 × dilution factor × 40 μg/ml = d-siRNA μg/ml.
2.3. d-siRNA transfection and gene silencing
For silencing endogenous MIF gene expression, wild-type AGN2a and AGN2a/CD80−137L cell lines were transferred into 48-well plates the day before transfection to ensure 50−70% confluency on the day of transfection. Initial transfections were performed to determine the optimal amounts of d-siRNA and GeneSilencer transfection reagent. 400−500 ng of d-siRNA in siRNA diluent was mixed with diluted GeneSilencer reagent and incubated for 5−10 min at room temperature. The mixture was transferred to the tumor cells in 200 μl of serum-free DMEM. 10% FBS was added 4 h after transfection, and fresh DMEM with 10% FBS was added as needed after 24 h. Cells and culture supernatants were collected at 60−72 h after transfection to assess MIF protein levels by Western blot and the other functional assays listed in the following sections.
2.4. MIF detection and quantification
Inhibition of macrophage migration was carried out as previously described [12]. Peritoneal macrophages from strain A/J mice were harvested by lavage with 5 ml serum-free DMEM 72 h following intraperitoneal (i.p.) injection with 0.5 ml of 3% peptone solution. 4 × 106 collected cells were pelleted by centrifugation, resuspended in a minimal volume of c-DMEM and added to microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, PA, heat-sealed using an open flame and then autoclaved) using a micropipette. Loaded capillary tubes were placed in 12 × 75 mm polystyrene tubes (Falcon) and macrophages pelleted by centrifugation. Capillary tubes were then wrapped in sterile gauze and snapped in half so as to lie flat in the bottom of 24-well plates containing 1 ml of media. Tubes were handled throughout with sterile forceps. Each assay was performed in triplicate wells containing a single capillary tube and cultured with culture supernatant from AGN2a or AGN2a cells transfected with MIF d-siRNA, GFP d-siRNA, or no plasmid (mock transfection) 60−72 h post-transfection. After 24 h of culture, the capillary tubes were removed and cells that had migrated out of the capillary tubes counted. MIF concentration in 72 h culture supernatants collected from 1 × 106 wild-type U2OS cells, U2OS cells transfected to over-express MIF (U2OS/MIF), or AGN2a cells was determined by ELISA using the Chemikine™ rat/mouse macrophage inhibitory factor (MIF) EIA kit from Chemicon International (Temecula, CA). Although MIF-containing culture supernatants could be frozen for ELISA analysis, MIF bioactivity rapidly degraded over a period of weeks. Supernatants used in functional assays did not contain antibiotics used for selection (i.e. Geneticin).
2.5. MIF Western blots
d-siRNA treated AGN2a cells were lysed 60−72 h after transfection in reducing loading buffer (NuPAGE system, Invitrogen, Carlsbad, CA), lysates subjected to SDS–PAGE, and resolved proteins transferred to PVDF membranes (Immun-Blot PVDF, 0.2 μm, Bio-Rad, Hercules, CA) using a NuPAGE Bis-Tris electrophoresis system (Invitrogen). Blots were probed with anti-mouse MIF polyclonal antibody (Abcam) at a 1:2500 dilution, followed by alkaline phosphatase conjugated secondary antibody (Abcam) at a 1:1000 dilution. CDP-star chemiluminescent reagent (Perkin–Elmer Life Science, Boston, MA) was used for AP detection.
2.6. T-cell activation assays
Splenocytes were collected from B6 (H-2b) mice and Thy1.2+ T lymphocytes purified using an AutoMACS apparatus after incubation with anti-Thy1.2-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA) for 30 min at 4 °C. For activation/proliferation experiments, 1 × 105 purified T cells were cultured in 96-well tissue culture plates. The T cells were activated with either: (a) 50 U/ml IL-2 (recombinant human, Proleukin, Chiron, Emeryville, CA) and 12.5 ng/ml IL-15 (recombinant mouse, R&D, Minneapolis, MN); (b) allogeneic AGN2a/CD80−137L (H-2a) tumor cells irradiated with 3000 rad prior to co-culture; or (c) immobilized anti-CD3 monoclonal antibody (Pharmingen, clone 145−2C11) (10 μg/ ml). 0.5 μCi [3H]thymidine (Perkin–Elmer Life Science) was added after 72−96 h of culture and cells harvested onto glass-fiber filters after an additional 18 h using an Inotech cell harvester (Rockville, MD). All assays were carried out in triplicate. For cytokine analysis T cells were isolated from B6 spleens by immunomagnetic bead sorting and cultured in IL-2 (50 U/ml) and IL-15 (12.5 ng/ml). Cytokine levels in culture supernatants were measured using the BD Cytometric Bead Array, Mouse Th1/Th2 cytokine kit (BD Biosciences Pharmingen). Assays performed with “activated T cells” were carried out following three days of culture with IL-2 and IL-15.
3. Results
3.1. In vitro demonstration of MIF knockdown
In previous work we described the production of soluble MIF in low passage cultures of patient-derived tumors and in a mouse neuroblastoma cell line, AGN2a [12]. To determine the functional consequences of inhibiting MIF production by AGN2a, endogenous MIF RNA was blocked with d-siRNA (dicer-generated small interfering RNA). d-siRNA was transfected into wild-type AGN2a, or an AGN2a-derived tumor vaccine cell line, AGN2a/CD80−137L (AGN2a permanently transfected with CMV-based mammalian expression vectors for both CD80 and CD137L) [24,25]. Loss of MIF protein expression was documented by Western blot of transfected cell lysates using a polyclonal antibody specific for murine MIF, Fig. 1A. Control experiments with d-siRNA specific for GFP and non-transfected cells did not show an equivalent loss of MIF. When supernatants from siRNA-treated cells were analyzed for MIF production by ELISA, a significant reduction in soluble MIF production was seen (Fig. 1B). Functional loss of MIF activity was demonstrated using the classical MIF assay, wherein the presence of MIF prevents migration of macrophages from a glass capillary tube. Peritoneal macrophages loaded in capillary tubes and co-cultured with supernatant from MIF-d-siRNA transfected (knockdown) AGN2a migrated out of the capillary tube faster and in greater numbers than those co-cultured with supernatants from untreated AGN2a (Fig. 1C). This important assay, using RNA interference, confirms that although other soluble mediators can inhibit random migration of macrophages, MIF itself is indeed responsible for this activity in tumor culture supernatants.
Fig. 1.
Loss of MIF expression by d-siRNA specific for MIF. (A) Cell lysates from AGN2a and AGN2a/CD80−137L cells that were untreated (lane 1), treated with transfection reagent alone (lane 2), transfected with d-siRNA specific for MIF (lane 3), or transfected with control d-siRNA specific for GFP (lane 4) using the GeneSilencer transfection system (described in Section 2) were prepared from cells collected 60−72 h after transfection and whole cell lysates fractionated by polyacrylamide gel electrophoresis. The electrophoresed proteins were transferred to membranes and detected with anti-MIF polyclonal antibody. (B) 2 × 104 AGN2a cells treated with transfection reagent alone (mock), treated with MIF-siRNA (MIF-siRNA), treated with GFP-siRNA, or untreated, AN2a, were cultured in 1 ml of c-DMEM for 48 h, supernatant collected and MIF concentration determined by ELISA. Average concentration and standard deviation from triplicate wells is shown. Only MIF-siRNA treated tumor showed significantly lowered MIF production when compared to mock transfection (P < 0.05, Student's paired t-test, used in all figures upon F-test for variance unless otherwise noted). (C) To demonstrate functional MIF activity, peritoneal macrophages loaded in capillary tubes were cultured in media alone (cntrl) or in day 3 culture supernatants from AGN2a cells (AGN2a), AGN2a cells transfected with d-siRNA for MIF (+MIF siRNA), AGN2a cells transfected with d-siRNA for GFP (+GFP siRNA), or from AGN2a cells treated with the transfection reagents in the absence of added nucleic acid (T-cntrl). Following 24 h of incubation in these culture supernatants, macrophage-loaded capillary tubes were removed from triplicate tissue culture wells and cells that had migrated out of the capillary tubes counted. Data plotted in all figures is the average and standard deviation from triplicate assay wells unless otherwise noted. Only control and MIF siRNA-treated tumor differed significantly from mock-transfected tumor (P < 0.05). The results are representative of three experiments.
3.2. Tumor-derived MIF blocks T-cell activation
We next explored the effect of neuroblastoma-derived MIF on T-cell activation in vitro. T cells isolated from B6 mice proliferated in vitro (as measured by [3H]thymidine incorporation) when cultured with IL-2 and IL-15 (Fig. 2A). If culture supernatant from the AGN2a/CD80−137L neuroblastoma cell line was added to the IL-2/IL-15-stimulated T-cell cultures, the incorporation of thymidine was inhibited. The ability of MIF-containing culture supernatant to inhibit T-cell proliferation was partially reversed by transfecting the tumor cells with MIF-specific d-siRNA, but not reversed by transfecting tumor cells with control d-siRNA complementary to GFP.
Fig. 2.
Tumor-derived MIF directly inhibits immune activation of T cells. (A) Purified T cells from B6 mice, 1 × 105, were incubated with 50 U/ml IL-2 and 12.5 ng/ml IL-15. [3H]Thymidine was added after 72−96 h of culture and the cells harvested after overnight thymidine incorporation. Incorporated average counts per minute (cpm) from triplicate wells (error bars indicate standard deviations) treated with cytokines only (cntrl) or wells containing dilutions of day 3 culture supernatants from non-transfected AGN2a (wild type), AGN2a transfected with d-siRNA for MIF (MIF siRNA), or AGN2a transfected with d-siRNA for GFP (GFP siRNA) are shown. Dilutions of the culture supernatants are indicated on the right of the figure. GFP and wild-type differed significantly from MIF si-RNA (P < 0.02) while the 1:100 dilution of MIF siRNA treated cell culture supernatant did not significantly differ from the control. (B) The amounts of MIF in culture supernatants from AGN2a (solid bars) and from U2OS/MIF cells were normalized by ELISA and then used in the same thymidine incorporation assay. Average thymidine incorporation and standard deviation of triplicate wells are shown, representative of three experiments. MIF from either source resulted in significant loss of proliferative activity (P < 0.01) at 10 pg/ml, while differences at 1 pg/ml were not statistically significant, demonstrating dose dependency.
In order to further clarify the role of MIF in the suppression of T-cell activation, we cloned MIF cDNA from AGN2a and used it to generate cell lines permanently transfected with CMV-based mammalian expression vectors. Numerous cell lines, including YAC-1, K562, and HEK293, were found to produce MIF as detected in culture supernatants or cell lysates by Western blot (not shown). The human osteosarcoma cell line U2OS did not secrete MIF and thus was used as a producer cell line for murine MIF. With the recent availability of an MIF ELISA assay we were able to quantify the levels of MIF produced by gene-transfected U2OS cells, now termed U2OS/MIF, as well as in AGN2a cells. Wild-type AGN2a cells produce even more MIF than the permanently transfected U2OS cell line, approximately 100 vs. 40 ng/ml at day 3 of culture, respectively (not shown). MIF concentrations in U2OS/MIF and AGN2a were normalized by dilution of the supernatants in media, and then dilutions giving the indicated MIF concentrations added to the same IL-2 and IL-15 driven lymphocyte proliferation assay. When concentrations were normalized U2OS/MIF and AGN2a tumor cell culture supernatants inhibited IL-2/IL-15-induced T-cell proliferation to a similar degree (Fig. 2B). When equivalent dilutions of wild-type (non-MIF producing) U2OS were assayed, no inhibition of proliferation was seen. These results demonstrate that MIF activity can be transferred to a heterologous cell lines, and when expressed as a transgene, MIF still inhibits T-cell activation.
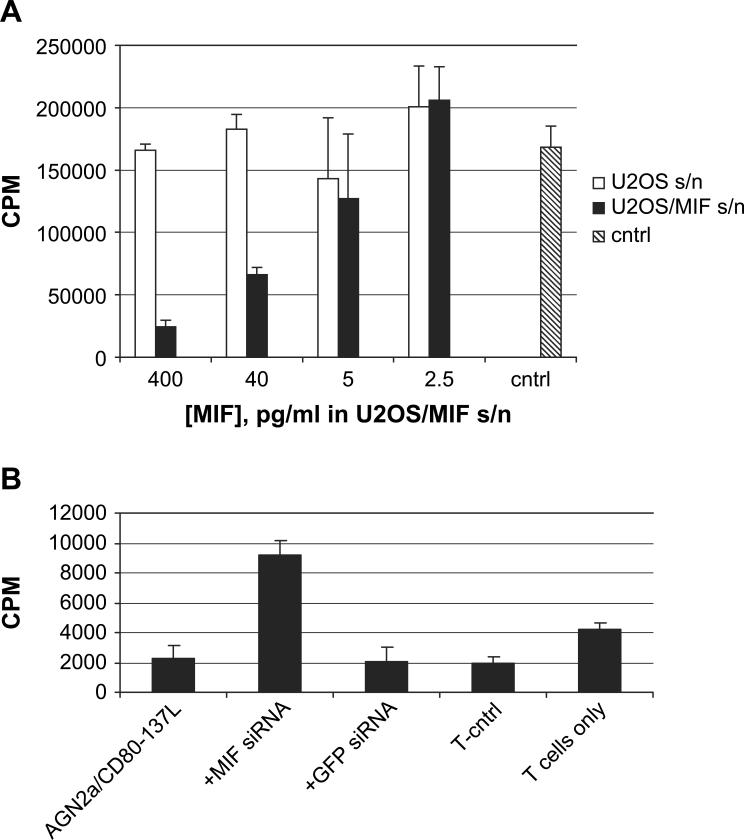
We next explored whether MIF could inhibit other T-cell activation pathways. When splenic T cells were activated by culture on anti-CD3 antibody coated tissue culture plates, MIF also inhibited activation in a dose-dependent manner (Fig. 3A). MIF in this assay was derived from U2OS/MIF supernatants and final MIF concentration determined by ELISA. In previous work we demonstrated that the AGN2a/CD80−137L tumor cells can serve as a cell-based tumor vaccine for murine neuroblastoma [25]. AGN2a/CD80−137L induces a strong T-cell dependent immune response that is able to protect vaccinated mice from challenge with live tumor. One aim of this previous work was to use AGN2a expressing CD80 and CD137L to induce specific T-cell expansion in vitro. However, we were not able to do so due to the apparent presence of soluble inhibitory factors. Experiments were then designed to explore if MIF plays a role in this inhibitory effect. We used T cells from B6 mice, and relied upon the MHC mismatch between the tumor line and the responding T cells to induce allogeneic proliferation (AGN2a is derived from strain A). Even with complete MHC disparity the AGN2a/CD80−137L ‘stimulator’ cells were unable to induce allogeneic T-cell activation, and in fact, the presence of irradiated tumor cells resulted in less thymidine incorporation than that seen with T cells cultured without stimulation (Fig. 3B; T-cntrl vs. T cells only). Transfection of AGN2a/CD80−137L cells with d-siRNA for MIF was able to reduce the suppression of T-cell expansion, as indicated by increased allogeneic T-cell thymidine incorporation (Fig. 3B). Thus, MIF inhibited three mechanisms of T-cell activation: cytokine, solid-phase anti-CD3, and allogeneic MHC.
Fig. 3.
Anti-CD3 and allo-immune activation is blocked by MIF. (A) T cells were stimulated with immobilized anti-CD3 antibody in the presence of serial dilutions of culture supernatants (s/n) from U2OS/MIF cells (closed bars) or wild-type U2OS cells (open bars). The concentrations of MIF listed on the axis are taken from ELISA analysis of U2OS/MIF supernatants. The wild-type U2OS supernatants (open) represent equivalent dilutions of supernatant, not MIF concentration. Control (gray) did not contain tumor cell culture supernatant. Averages and standard deviations of triplicate wells are shown. Cells were cultured for 48 h, pulsed with [3H]thymidine and harvested on day 3. In this experiment only 400 and 40 pg/ml MIF differed significantly from control (P < 0.01). (B) Allogeneic T-cell proliferation was induced by co-culturing 1 × 105 T cells from B6 mice with 1 × 104 irradiated strain A-derived AGN2a/CD80−137L cells, AGN2a/CD80−137L transfected with d-siRNA for MIF (+MIF siRNA), AGN2a/CD80−137L transfected with d-siRNA for GFP (+GFP siRNA), or AG-N2a/CD80−137L treated with transfection reagents alone (T-cntrl). Thymidine incorporation by T cells cultured without irradiated tumor cells is also shown (T cells only). [3H]TdR was added 96 h after initiation of co-culture and triplicate wells harvested after overnight incubation. The data shown in each plot are representative of three independent experiments. In comparison to T cells cultured with AGN2a/CD80−137L cells, MIF siRNA treatment differed significantly (P < 0.02), while GFP siRNA or transfection control did not. T cells cultured alone did incorporate thymidine (P < 0.04 compared to AGN2a/CD80−137L) illustrating the inhibitory effects of tumor cells in the assay.
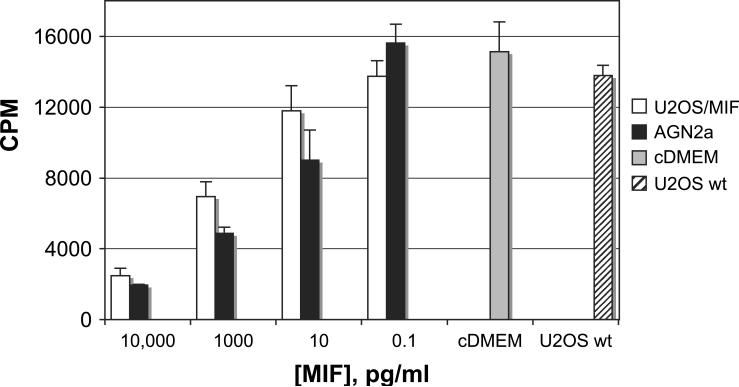
The inhibition of T-cell activation by MIF was interesting in itself, but perhaps even more applicable to MIF's role as an immune evasion molecule is the ability to block expansion of T cells that have already been activated. We activated splenic T cells for three days with IL-2 and IL-15, and then introduced MIF from U2OS/MIF and AGN2a culture supernatants (Fig. 4). As before, the level of MIF in the culture supernatants was determined by ELISA and diluted accordingly. As with addition of MIF at the time of activation, MIF also blocked the expansion of T cells that were already activated and expanding in number. Control supernatants from non-MIF expressing U2OS showed thymidine incorporation similar to media and cytokine only controls (not shown).
Fig. 4.
MIF inhibits activated T cells. Splenic T lymphocytes were cultured for 72 h in IL-2 and IL-15, as before. U2OS/MIF (open) or AGN2a (filled) culture supernatant with MIF concentrations normalized by ELISA was then added on day 3, and the cultures re-fed with fresh IL-2 and IL-15 at this time. The cultures were also re-fed with fresh IL-2 and IL-15 at this time. [3H]thymidine was added on day 6 and cells harvested to determine thymidine uptake on day 7. Thymidine incorporation of cells cultured in cytokines and diluted U2OS wild-type supernatant or media (U2OS wt, hatched, cDMEM, gray) is also shown. Average counts from three wells and standard deviations are shown. In comparison to cDMEM control, significant differences were only seen for AGN2a-derived supernatant containing 10 pg/ml MIF (AGN2a P < 0.02, U2OS/MIF P = 0.058), while both supernatants differed significantly at 1000 pg/ml MIF (P < 0.01). Data are representative of two experiments.
3.3. Tumor-derived MIF induces cell death in activated cultures
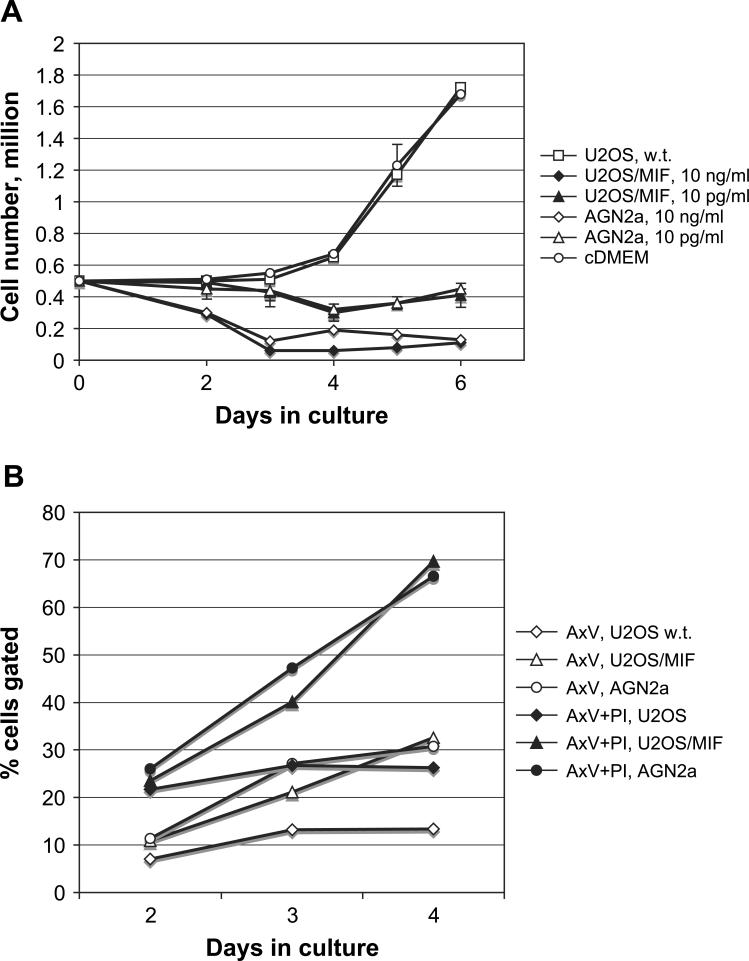
Upon microscopic inspection of IL-2/IL-15 and MIF treated T cells, the lack of thymidine incorporation by MIF-treated cells was surprising as blast-like cells could be seen in these cultures. Therefore, in addition to thymidine incorporation assays we also counted the total cell number in culture. At day 2, low levels of MIF (10 pg/ml) did not significantly impact cell number, but a slight decrease with high concentration of MIF (10 ng/ml) could be detected (Fig. 5A). By day 4, T cells stimulated with IL-2 and IL-15 began to rapidly expand in cell number while the cell number in cultures that also contained MIF fell. When analyzed by flow cytometry, apoptotic cells were detected by annexin V staining, and late apoptotic/necrotic cells detected by co-staining with annexin V and propidium iodide (Fig. 5B). A steady increase in dying cells that were positive for both PI permeability and annexin V binding (A × V + PI) could be seen on days 2, 3, and 4 when cells were co-cultured with IL-2, IL-15 and MIF. When cultured cells were gated for annexin V binding alone, MIF-containing supernatant (U2OS/MIF and AGN2a) showed an increase in gated cells when compared to cells cultured in an equivalent amount of control culture supernatant (U2OS).
Fig. 5.
MIF inhibits the numeric expansion of cytokine activated T cells. (A) B6 T cells were cultured with IL-2 and IL-15 in the absence of culture supernatants (cDMEM, open circle), in the presence of U2OS/MIF supernatant diluted to 10 ng/ml or 10 pg/ml MIF as determined by ELISA (closed diamond and closed triangle, respectively), AGN2a supernatant diluted to 10 ng/ml or 10 pg/ml (open diamond and open triangle, respectively) or with wild-type U2OS supernatant at the same dilution as the U2OS/MIF supernatant (open square). Data shows total viable cells in culture as determined by vital dye exclusion. Values plotted are the average of triplicate wells, and standard deviations greater than 10% are shown. 10 ng/ml MIF from either source differed significantly from U2OS control on day 2 (P < 0.01), and at 10 ng/ml on day 4 (P < 0.01). (B) Cultured T cells were analyzed by flow cytometery upon exposure to culture supernatants. Open symbols show the percentage of cells at the indicated time points that stained positive with annexin V and the closed symbols show the percentage of cells that gated positive for both annexin V and propidium iodide (PI) staining. Cells were treated with 10 ng/ml MIF from AGN2a culture supernatant (circles), U2OS/MIF culture supernatant (triangles), or media alone (diamonds).
By day 4, when a clear decrease in cell number could be seen in MIF treated cells, the majority of these cells were both annexin V and PI positive, indicating that as cells become annexin V positive they progressed to a late apoptotic phenotype and cell death.
3.4. IFN-γ production by MIF treated T cells
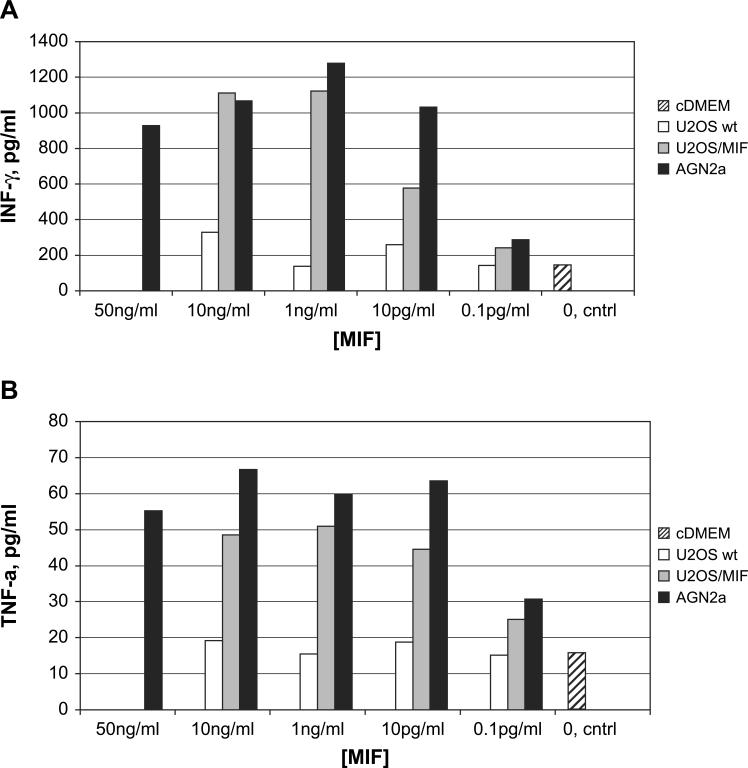
The increased apoptosis seen in MIF-treated T-cell cultures led us to examine the supernatants of treated T cells for other cytokines that may influence T-cell activation. IL-2 and IL-15 treated T cells were cultured in the presence or absence of MIF, and the resulting culture supernatants analyzed for the presence of IL-2, IL-4, IL-5, IFN-γ, and TNF-α using a flow cytometric bead array. Results from the analysis of 72 h culture supernatants for IFN-γ (A) and TNF-α (B) are shown in Fig. 6. As the IL-2 source in our assays is human, we were able to test for elevation in murine IL-2 as well. The only cytokines that showed differences over the media-only background were IFN-γ and TNF-α. The IFN-γ induction by MIF was remarkable, and both AGN2a and U2OS/MIF supernatants induced concentrations of IFN-γ exceeding 1 ng/ml. The differences in TNF-α levels were much smaller (Fig. 6B), but still the increase caused by the addition of MIF was clear.
Fig. 6.
Analysis of cytokines produced by MIF-treated T cells. B6 T cells were cultured in the presence of IL-2 and IL-15 alone (striped) or with control U2OS (open), U2OS/MIF (gray), or AGN2a (black) culture supernatants normalized to the indicated MIF concentrations by ELISA. After 72 h of T-cell culture, IFN-γ (panel A) and TNF-α (panel B) levels in the culture supernatants were determined using a flow cytometric bead array. A representative experiment of more than three independent experiments is shown.
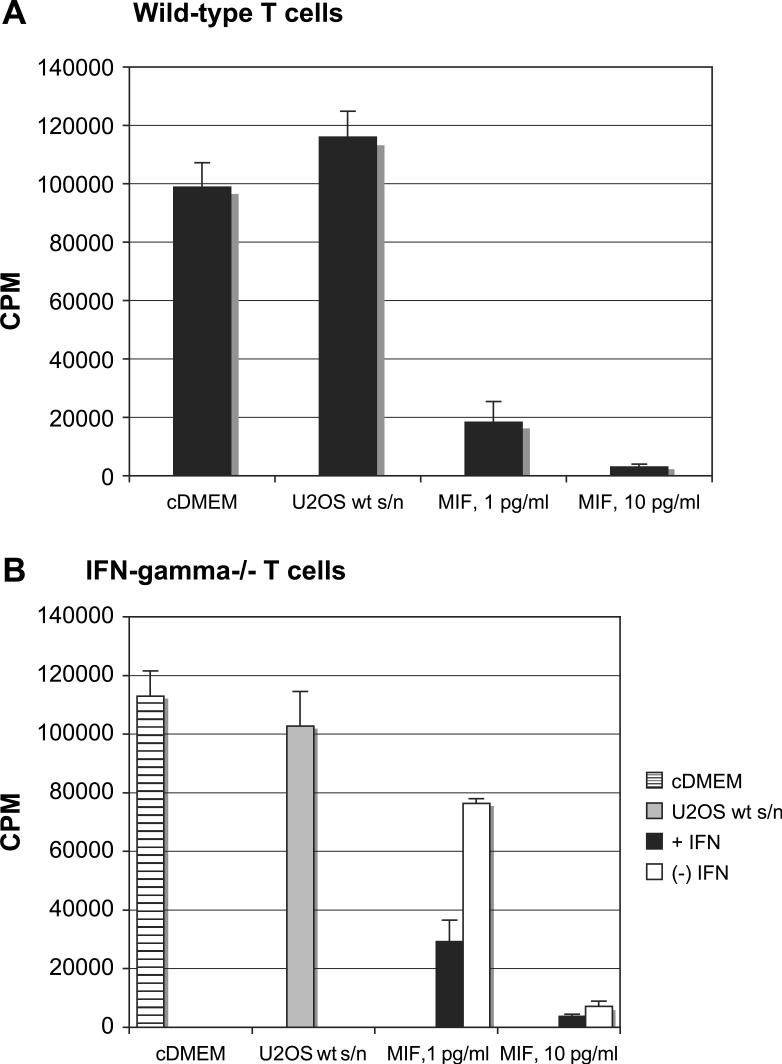
3.5. MIF effects on T cells from IFN-γ knockout mice
To make a more direct connection between the high MIF-induced IFN-γ levels and MIF-inhibited T-cell activation, IFN-γ knockout mice were used as the source of responding T cells. When IL-2/IL-15-stimulated T lymphocytes were treated with MIF-containing supernatants (U2OS/MIF), lymphocytes from IFN-γ knockout mice were inhibited far less than those from wild-type mice (Fig. 7). Wild-type cells from B6 mice lost proliferative capacity in response to MIF derived from MIF-transfected U2OS cells (Fig. 7A). Differences between cDMEM media control and supernatants from wild-type U2OS cells (diluted to the same degree as the highest concentration of MIF supernatant) were not statistically significant for B6 T cells or T cells from IFN-γ −/− mice. While MIF-treated IFN-γ −/− T cells were significantly inhibited (P = 0.02) as compared to control supernatant, the inhibition was far less than that seen with wild-type cells. At 1 pg/ml MIF thymidine incorporation decreased by 84% in B6 T cells with reference to control, while the decrease in IFN-γ −/− mice was only 26% (drop of 98,145 cpm as opposed to 26,247 cpm, respectively). To further establish the participation of IFN-γ in our assay, recombinant IFN-γ was added to knock-out lymphocytes, and was shown to significantly decrease thymidine incorporation at both 1 pg/ml and 10 pg/ml of MIF (P < 0.01 and P < 0.032, respectively) (Fig. 7B). This establishes IFN-γ as an important contributor to the MIF-mediated inhibition of T-cell activation. Similar results were also seen with culture supernatants from AGN2a (not shown). Although IFN-γ knockout mice did not completely restore proliferative capacity, we can conclude that the effects of MIF on activated T cells are complex and include production of soluble factors like IFN-γ and TNF-α that result in autocrine modulation of T-cell expansion and viability.
Fig. 7.
IFN-γ contributes to MIF effects on T cells. T cells from (A) wild-type (wt) or (B) IFN-γ knockout B6 mice were cultured with IL-2 and IL-15 as described previously. MIF, derived from culture supernatants of U2OS/MIF cells, was added at the indicated concentrations. Positive controls consisted of wells containing no tumor cell culture supernatant (cDMEM) or supernatants from the parental U2OS cell line at a dilution that matched the dilution of U2OS/MIF. [3H]Thymidine incorporation was assessed after 3 days in culture. Average counts from triplicate wells and standard deviations are shown. Results are representative of three experiments. In panel B, recombinant mouse IFN-γ was added at 1 μg/ml (filled) or not added (open) at the beginning of the assay as indicated.
4. Discussion
During our study of the in vitro immune response to neuroblastoma we found that murine neuroblastoma cell lines inhibited rather than stimulated T lymphocyte activation in vitro. Unexpectedly, we determined that MIF is in large part the responsible agent. The MIF produced by neuroblastoma is secreted at super-physiological levels, exceeding 100 ng/ml in 3-day cultures of AGN2a. To date, MIF was thought to promote T-cell immunity as antibody depletion studies showed a critical requirement for MIF in T-cell activation, with the maximal levels of MIF being produced at 15 ng/ml [8]. At 24 h after anti-CD3 activation of lymphocytes, the levels of MIF in culture supernatants were 5 ng/ml, and they climbed only to 15 ng/ml by day 2 in those studies. When PMA was used to activate T cells, the 12, 24, and 48 h culture time points showed MIF concentrations of 10, 15, and 10 ng/ml, respectively. In other studies with mouse T-cell lines, a Th1 line did not produce any MIF, while a Th2 line produced approximately 8 ng/ml. Thus it was proposed that these levels of MIF produced by lymphocytes may steer the immune system towards Th2 responses [8]. If the levels produced by AGN2a (100 ng/ml at 72 h of culture) are similarly produced in vivo this would constitute a continuous exposure of high levels of MIF in the tumor environment, as opposed to low-level regulated responses. A further level of complexity in analyzing MIF activity is its lability. We found that even when MIF-containing supernatants were stored at −80 °C, there was a significant depreciation of MIF activity. While our supernatants were normalized to the MIF concentrations obtained by ELISA, they were not normalized for function. We found that distinct differences in MIF activity could be seen in culture supernatants stored for even short periods of time when compared to the activity present in fresh tumor cell supernatants. We note these parameters in order to highlight the care that must be taken in comparing functional assays of MIF in different systems.
What is the potential impact of high levels of MIF production? Levels of pituitary-secreted MIF in the serum following LPS administration to mice are higher than those measured in lymphocyte assays in vitro, reaching 50−500 ng/ml [5]. At these levels MIF is toxic. For MIF to override the effect of glucocorticoids on lymphocytes, concentrations of 1−10 ng/ml are optimal. Calandra et al. demonstrated that MIF released by LPS or tetanus toxoid (TSST-1) treated macrophages follows a bell-shaped curve. MIF production was turned off at higher concentrations of the stimulatory agents, perhaps to protect against the detrimental effects of excessive MIF release [26]. Thus, the levels of physiological MIF production are tightly regulated.
From a tumor-oriented perspective, MIF production provides an obvious advantage as it promotes escape from p53-mediated growth control [27]. In an in vivo tumor model (OVA-transfected EL-4), splenocytes from immunized mice secreted high levels of MIF following antigenic stimulation in vitro, and neutralizing anti-MIF antibody treatment of these cultures elicited a significant increase in CTL response, as well as increased levels of IFN-γ [23]. In this study, MIF production was not beneficial to the development of a T-cell immune response. Anti-MIF antibody treatment has been shown to amplify T-cell responses in three tumor model systems: a syngeneic B cell lymphoma model, mice bearing a human melanoma, and mice bearing EG.7 lymphoma [23,28,29]. MIF neutralization promoted CTL activity, and in some cases inhibited tumor growth and vascularization. These data indicate that in tumor-bearing animals, MIF plays a dual role in both promoting tumor cell growth and inhibiting T-cell responses.
Our studies with neuroblastoma support other reports regarding prostatic adenocarcinoma, glioblastoma multiformae, and lung adenocarcinoma where the production of MIF by tumor exceeded the levels produced by non-transformed cells or tissues [28–31]. The levels of MIF produced in culture by seven primary uveal melanoma lines ranged from 25 to 50 ng/ml, while two metastatic lines produced 100−150 ng/ml [32]. The only way to rectify the dichotomy between the activation of T cells in some systems, and the seemingly pro-oncogenic activity in others is to propose that the combination of high MIF levels and continuous exposure to MIF inhibits cellular immunity. Our studies demonstrate that MIF produced by tumor cell lines has a profound ability to blunt T lymphocyte responses. This includes T-cell activation by the cytokines IL-2 and IL-15, by solid-phase anti-CD3, and by allogeneic stimulation (Figs. 2 and 3).
Ren et al. confirmed our earlier work describing the presence of MIF in murine and human neuroblastomas [33]. This group also provided evidence that MIF is upregulated in neuroblastoma tumor tissues and cell lines, and that MIF expression appears to correlate with the degree of tumor differentiation. Furthermore, they found that MIF caused a dose dependent increase in VEGF and IL-8 secretion, and that increased MIF also correlated with MYCN expression in neuroblastoma tissues. In their report, N-Myc translocation was dependent on ERK signaling pathways, and the inhibition of ERK reduced MIF-mediated N-Myc expression. Thus, MIF induces N-Myc expression and it also increases angiogenic factors that potentiate tumor cell growth. Nuclear localization of N-Myc may be important for dimerization with its binding partner Max and for activation of other growth-promoting genes in tumors [34].
Work done by Ishizaka and colleagues demonstrated that glycosylation inhibition factor (GIF) and MIF are one in the same protein. The sole difference is the cysteinylation of Cys-60 in MIF, thus converting it to GIF. This unique post-translational modification was found to be responsible for the activity of a human suppressor T-cell hybridoma that suppresses the IgE antibody response [35]. The modified Cys-60 MIF/GIF did not induce TNF-α production from the RAW 264.7 macrophage cell line as MIF does, but GIF does bind to activated T and B cells [36]. Thus, the possibility exists that MIF secreted by tumors could be post-translationally modified and display an entirely novel spectrum of physiologic activity.
In our studies, MIF induced the production of high levels of IFN-γ from activated T lymphocytes, and the IFN-γ was responsible, at least in part, for activation-induced cell death (Figs. 5 and 7). Interferon-γ causes cell growth arrest at the G1 phase of the cell cycle [37]. The biological effects of interferon with respect to growth inhibition and apoptosis are mediated by the Jak/STAT pathway, with STAT-1 being the principal signaling molecule responsible for the expression of p21Waf-1/Cip1 and IRF-1 [38–40]. In response to IFN-γ stimulation, STAT-1 (signal transducer and activator of transcription) becomes phosphorylated and forms a homodimer through reciprocal SH2-phosphotyrosine interactions, and it is subsequently translocates to the nucleus where DNA binding and gene activation occur [41,42]. STAT-1 signaling is able to upregulate multiple genes involved in apoptosis such as caspases, Fas, FasL and the cdk inhibitors p21Waf1 and p27kip1 [43–45]. Even thought the role of STAT-1 in the regulation of apoptosis following various stress-induced stimuli is well documented, and lymphocytes deficient in STAT-1 show reduced apoptosis and enhanced proliferation, the precise molecular mechanism of how STAT-1 mediates apoptosis is unclear [43,46,47].
Excessive antigen density may result in T-cell receptor induced depletion of T effector cells, called Ag-induced cell death (AgICD). CD3/TCR signaling activates STAT5 and when this signaling leads to apoptosis, it is dependant on Fas/FasL interactions [48,49]. IL-15 exacerbates apoptosis in CD4+ T cells activated with anti-CD3 and anti-CD28, potentially by recruiting more blasts capable to die [50]. AgICD requires Fas/FasL interactions, does not require perforin or granzymes, and TNF-α is thought to play a late role in the process [51]. Antibodies to IFN-γ inhibit AgICD, and thus the executor molecules FasL or TNF-α facilitate a process that is initiated by IFN-γ [51]. Thus, the activation of cytokine receptors may have two opposing effects, the activation of MAPs or other signal such as PI3 kinase pathway that promote growth and survival or a STAT pathway that leads to cell cycle arrest or death. In this context the activity of MIF would seem to initiate the IFN-γ release that leads to cytokine-mediated activation induced cell death.
The growth promoting effect of MIF on tumors appears to be dependent on numerous cell activation pathways as well as loss of p53 cell cycle control. The finding that MIF is secreted by murine tumors as well as human cancers suggests that MIF secretion may be “selected for” in developing tumors that can express it. Recent data generated using both anti-sense strategies to knock-down MIF or MIF transgenic mice indicate that MIF may promote tumor growth and angiogenesis early in the growth phase of the tumor, while still mediating some levels of immune stimulation as the tumor progresses [52,53]. Our data suggest that high levels of tumor-derived MIF target activated tumor-specific lymphocytes for further activation through STAT-1/IFN-γ mediated pathways leading to their death. These high and continuous levels of MIF production operate in opposition to the natural MIF production seen in autoimmune disease models. In these models, inhibition of MIF with antibody, with the pharmacologic agent ISO-1, or when disease is modeled in MIF −/− mice, abrogation of MIF inhibits immune activation [54–56]. However, in our tumor model a naturally occurring cytokine that can activate the immune system at physiological concentrations becomes an active inhibitor of the immune response when expressed at high levels.
Acknowledgments
We would like to thank Natalia Natalia for technical support, and acknowledge support from the Midwest Athletes Against Childhood Cancer (MACC Fund, Inc.) and from National Institutes of Health, NCI grant CA100030.
References
- 1.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56:72–7. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett B, Bloom BR. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci USA. 1968;59:756–62. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhagen J, Calandra T, Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med. 1998;76:151–61. doi: 10.1007/s001090050204. [DOI] [PubMed] [Google Scholar]
- 4.Weiser WY, Temple PA, Witek-Gianotti JS, Remold HG, Clark SC, David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1989;86:7522–6. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 6.Kleeman R, Rorsman H, Rosengren E, Mischke R, Mai NT, Bernhagen J. Dissection of the enzymatic and immunologic functions of macrophage migration inhibitory factor. Eur J Biochem. 2000;267:7183–92. doi: 10.1046/j.1432-1327.2000.01823.x. [DOI] [PubMed] [Google Scholar]
- 7.Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA. 1992;1992(89):3511–5. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhagen J, Bacher M, Calandra T, Metz CN, Doty SB, Donnelly T, et al. An essential role for macrophage migration inhibitory factor in tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277–82. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsuhashi M, Liu J, Cao S, Shi X, Ma X. Regulation of interleukin-12 gene expression and its anti-tumor activities by prostaglandin E2 derived from mammary carcinomas. J Leukoc Biol. 2004;76:322–32. doi: 10.1189/jlb.1203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers WH, Rabinowich H, Herberman RB. Tumor associated immunodeficiency and implications for tumor development and prognosis. In: Kufe D, Pollack R, Weichselbaum R, Bast R Jr., Gansler T, Holland J, et al., editors. Cancer medicine 6. BC Decker Inc.; Hamilton: 2003. p. electronic-resource. [Google Scholar]
- 12.Bin Q, Johnson BD, Schauer DW, Casper JT, Orentas RJ. Production of macrophage migration inhibitory factor by human and murine neuroblastoma. Tumor Biol. 2002;23:123–9. doi: 10.1159/000064028. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer FA, Pantschenko AG, Miller LJ, Anderson K, Grunnet M, KcKenna PH, et al. Angiogenesis and neuroblastomas: interleukin-8 and interleukin-8 receptor expression in human neuroblastoma. J Urol. 2000;164:1016–20. doi: 10.1097/00005392-200009020-00024. [DOI] [PubMed] [Google Scholar]
- 14.Satoh J, Kurohara K, Yukitake M, Kuroda Y. Interleukin-15, a T-cell growth factor, is expressed in human neural cell lines and tissues. J Neurol Sci. 1998;155:170–7. doi: 10.1016/s0022-510x(97)00310-9. [DOI] [PubMed] [Google Scholar]
- 15.Knezevic-Cuca J, Stansberry KB, Johnston G, Zhang J, Keller ET, Vinik AI, et al. Neurotrophic role of interleukin-6 and soluble interleukin-6 receptros in N1E-115 neuroblastoma cells. J Neuroimmunol. 2000;102:8–16. doi: 10.1016/s0165-5728(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 16.Condorelli F, Sortino MA, Stella AM, Canonico PL. Relative contribution of different receptor subtypes in the response of neuroblastoma cells to tumor necrosis factor-alpha. J Neurochem. 2000;75:1172–9. doi: 10.1046/j.1471-4159.2000.0751172.x. [DOI] [PubMed] [Google Scholar]
- 17.Pastrana DV, Raghavan N, Fitzgerald P, Eisinger SW, Metz C, Bucala R, et al. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–63. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermanowski-Vosatka A, Mundt SS, Ayala JM, Goyal S, Hanlon WA, Czerwinski RM, et al. Enzymatically inactive macrophage migration inhibitory factor inhibits monocyte chemotaxis and random migration. Biochemistry. 1999;38:12841–9. doi: 10.1021/bi991352p. [DOI] [PubMed] [Google Scholar]
- 19.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, et al. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci USA. 2003;100:9354–9. doi: 10.1073/pnas.1533295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;187:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleeman R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211–6. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 22.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe R, Peng T, Sailors J, Bucala R, Metz CN. Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol. 2001;166:747–53. doi: 10.4049/jimmunol.166.2.747. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BD, Yan X, Schauer DW, Orentas RJ. Dual expression of CD80 and CD86 produces a tumor vaccine superior to single expression of either molecule. Cell Immunol. 2003;222:15–26. doi: 10.1016/s0008-8749(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 25.Yan X, Johnson BD, Orentas RJ. Murine CD8 lymphocyte expansion in vitro by artificial antigen-presenting cells expressing CD137L (4−1BBL) is superior to CD28, and CD137L expressed on neuroblastoma expands CD8 tumor-reactive effector cells in vivo. Immunology. 2004;112:105–16. doi: 10.1111/j.1365-2567.2004.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci USA. 1998;95:11383–8. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–82. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264:751–8. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- 29.Kamimura A, Kamachi M, Nishihira J, Ogura S, Isobe H, Dosaka-Akuta H, et al. Intracellular distribution of macrophage migration inhibitory factor predicts the prognosis of patients with adenocarcinoma of the lung. Cancer. 2000;89:334–41. [PubMed] [Google Scholar]
- 30.Meyer-Siegler K, Hudson PB. Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology. 1996;48:448–52. doi: 10.1016/S0090-4295(96)00207-5. [DOI] [PubMed] [Google Scholar]
- 31.Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, et al. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- 32.Repp AC, Mayhew ES, Apte S, Niederkorn JY. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J Immunol. 2000;165:710–5. doi: 10.4049/jimmunol.165.2.710. [DOI] [PubMed] [Google Scholar]
- 33.Ren Y, Chan HM, Li Z, Lin C, Nicholls J, Chen CF, et al. Upregulation of macrophage migration inhibitory factor contributes to induced N-myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene. 2004;23:4146–53. doi: 10.1038/sj.onc.1207490. [DOI] [PubMed] [Google Scholar]
- 34.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;1999(17):2264–79. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 35.Watari H, Nozawa R, Tokunaga A, Yuyama N, Tomas M, Hinohara A, et al. Posttranslational modification of the glycosylation inhibiting factor (GIF) gene product generates bioactive GIF. Proc Natl Acad Sci USA. 2000;97:13251–6. doi: 10.1073/pnas.230445397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugie K, Nakano T, Tomura T, Takakura K, Mikayama T, Ishizaka K. High-affinity binding of bioactive glycosylation-inhibiting factor to antigen-primed T cells and natural killer cells. Proc Natl Acad Sci USA. 1997;94:5278–83. doi: 10.1073/pnas.94.10.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr Transcriptionally active STAT1 is required for the antiproliferative effects of both interferon α and interferon γ. Proc Natl Acad Sci USA. 1996;1996(93):7673. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin YE, Kitagawa M, Su W-CS, You Z-H, Iwamoto Y, Fu X-Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21WAF1/CIP1 mediated by STAT1. Science. 1996;1996(272):719–22. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 39.Lee KY, Anderson E, Madami K, Rosen GD. Loss of STAT1 expression confers resistance to IFN-γ-induced apoptosis in ME180 cells. FEBS Lett. 1999;459:323–6. doi: 10.1016/s0014-5793(99)01283-1. [DOI] [PubMed] [Google Scholar]
- 40.Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 41.Stephanou A, Latchman DS. STAT-1: a novel regulator of apoptosis. Int J Exp Pathol. 2003;84:239–224. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonard WJ, O'Shea JJ. JAKs and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 43.Lee C-K, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-γ. J Immunol. 2000;164:1286–92. doi: 10.4049/jimmunol.164.3.1286. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal S, Agarwal ML, Chatterjee-Kishore M, Stark GR, Chisolm GM. Stat1-dependent, p53-independent expression of p21waf1 modulates oxysterol-induced apoptosis. Mol Cell Biol. 2002;22:1981–92. doi: 10.1128/MCB.22.7.1981-1992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu X-Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J Biol Chem. 2004;279:5811–20. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 47.Sironi JJ, Ouchi T. STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J Biol Chem. 2004;279:4066–74. doi: 10.1074/jbc.M307774200. [DOI] [PubMed] [Google Scholar]
- 48.Welte T, Leitenberg D, Dittel BN, Al-ramadi BK, Xie B, Chin YE, et al. STAT5 interaction with the T-cell receptor complex and stimulation of T-cell proliferation. Science. 1999;283:222–5. doi: 10.1126/science.283.5399.222. [DOI] [PubMed] [Google Scholar]
- 49.Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- 50.Lin SJ, Yu J-C, Cheng P-J, Hsiao S-S, Kuo M-L. Effect of interleukin-15 on anti-CD3/anti-CD28 induced apoptosis of umbilical cord blood CD4+T cells. Eur J Haematol. 2003;71:425–32. doi: 10.1046/j.0902-4441.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 51.Sobek V, Balkow S, Körner H, Simon MM. Antigen-induced cell death of T effector cells in vitro proceeds via the Fas pathway, requires endogenous interferon-γ and is independent of perforin and granzymes. Eur J Immunol. 2002;32:2490–9. doi: 10.1002/1521-4141(200209)32:9<2490::AID-IMMU2490>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki Y, Kasuya K, Nishihira J, Magami Y, Tsichida A, Aoki T, et al. Suppression of tumor growth through introduction of an antisense plasmid of macrophage migration inhibitory factor. Int J Mol Med. 2002;10:579–83. [PubMed] [Google Scholar]
- 53.Fukushima T, Nishihira J, Yoshiki T, Iwabuchi K, Iwabuchi C, Yamasaki Y, et al. Evidence of dual function of macrophage migration inhibitory factor relevant to tumor progression and regression. Int J Mol Med. 2005;16:119–26. [PubMed] [Google Scholar]
- 54.Nicoletti F, Creange A, Orlikowski D, Bolgert F, Mangano K, Metz C, et al. Macrophage migration inhibitory factor (MIF) seems crucially involved in Guillain-Barre syndrome and experimental allergic neuritis. J. Neuroimmunol. 2005;168:168–74. doi: 10.1016/j.jneuroim.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Powell ND, Papenfus TL, McClain MA, Gienapp IE, Shawler TM, Satoskar AR, et al. Cutting edge: macrophage migration inhibitory factor in necessary for progression of experimental autoimmune encephalitis. J Immunol. 2005;175:5611–4. doi: 10.4049/jimmunol.175.9.5611. [DOI] [PubMed] [Google Scholar]
- 56.Cvetkovic I, Al-Abed Y, Miljkovic D, Maksimovic-Ivanic D, Roth J, Bacher M, et al. Critical role of macrophage migration inhibitory factor in experimental autoimmune diabetes. Endocrinology. 2005;146:2942–51. doi: 10.1210/en.2004-1393. [DOI] [PubMed] [Google Scholar]