Abstract
The antitumor effect of adoptively transferred tumor-specific cytotoxic T lymphocytes (CTLs) is impaired by the limited capacity of these cells to expand within the tumor microenvironment. Administration of interleukin 2 (IL-2) has been used to overcome this limitation, but the systemic toxicity and the expansion of unwanted cells, including regulatory T cells, limit the clinical value of this strategy. To discover whether transgenic expression of lymphokines by the CTLs themselves might overcome these limitations, we evaluated the effects of transgenic expression of IL-2 and IL-15 in our model of Epstein Barr Virus–specific CTLs (EBV-CTLs). We found that transgenic expression of IL-2 or IL-15 increased the expansion of EBV-CTLs both in vitro and in vivo in a severe combined immunodeficiency disease (SCID) mouse model and enhanced antitumor activity. Although the proliferation of these cytokine genes transduced CTLs remained strictly antigen dependent, clinical application of this approach likely requires the inclusion of a suicide gene to deal with the potential development of T-cell mutants with autonomous growth. We found that the incorporation of an inducible caspase-9 suicide gene allowed efficient elimination of transgenic CTLs after exposure to a chemical inducer of dimerization, thereby increasing the safety and feasibility of the approach.
Introduction
Adoptive transfer of antigen-specific cytotoxic T lymphocytes (CTLs) has shown efficacy in some patients with melanoma,1,2 Hodgkin lymphoma,3,4 and nasopharyngeal carcinoma.5,6 However, the antitumor activity of adoptively transferred CTLs is hampered by the limited capacity of these cells to significantly expand within the tumor microenvironment.7 The development of strategies to overcome this restriction could significantly improve the clinical outcome of patients receiving adoptive T-cell therapy.
Since adoptively transferred antigen-specific CTLs are highly dependent on exogenous cytokines for their continued growth and survival,8 systemic administration of interleukin 2 (IL-2) has been used to enhance their in vivo expansion and persistence.1 However, the prolonged administration of IL-2 is often associated with serious side effects, limiting the amount and duration of cytokine administration.1 Moreover, the effects of systemically administered cytokines are nonselective. IL-2 may favor the expansion of unwanted cell subsets, such as regulatory T cells,9 that constitutively express the IL-2 receptor and adversely affect the function of antitumor CTLs.10–12
Genetic manipulation of CTLs to express growth cytokines such as IL-2 and IL-15 could make them less helper-cell dependent and better able to sustain their proliferation and activation after antigenic stimulation.13,14 However, the constitutive expression of transgenes that enhance growth raises the concern that the T cells may lose antigen specificity and growth dependence and become growth autonomous.15 This is a particular concern where retroviruses are used to ensure transgene integration and thereby obtain cytokine secretion by the progeny of the modified cells.16,17 We therefore used our Epstein Barr virus (EBV)+ tumor model and EBV-specific CTLs (EBV-CTLs) to evaluate the biological effects of transgenic expression of IL-2 or IL-15, in association with transfer of a suicide gene based on an inducible caspase-9 (iCasp-9) protein18 that can be activated using a specific chemical inducer of dimerization (CID), analogs of which have been safely tested in a phase I study.19
We found that both transgenic IL-2 and IL-15 sustained CTL expansion and function in vivo and that these cytokine-gene modified cells retained antigen specificity and dependence. Activation of the iCasp-9 gene with CID efficiently ablated cytokine production and eliminated adoptively transferred T cells, suggesting that this combined approach could safely augment the efficacy of adoptively transferred tumor-specific CTLs.
Materials and methods
Human and animal studies were approved by the Institutional Review Board of Baylor College of Medicine.
Plasmid construction and retrovirus production
CD34 was used as a selectable marker of transduced cells. Full-length human CD34 (NCBI AF523361) was cloned by polymerase chain reaction (PCR) using clone ID 4746591 (Invitrogen, Carlsbad, CA). We truncated the cytoplasmic tail of CD34, 20 amino acids (YYT) downstream from the last amino acid of the putative transmembrane domain. This truncation was based on preliminary experiments showing stable expression of ΔCD34 in Jurkat cells (> 1 mo; data not shown) and the efficient selection of transduced T cells using anti-CD34 microbeads (Miltenyi, Bergisch Gladbach, Germany), a method approved for clinical use (CliniMacs). The full-length human IL2 and IL15 genes were cloned by PCR from plasmids obtained from InVivogene (San Diego, CA). The human IL15 gene encodes the isoform with the long 48 AA signal peptide.20 The construction of the iCasp-9 suicide gene was previously reported.18 Briefly, Caspase-9 gene is deleted for the caspase recruitment domain (CARD) domain and fused in frame with a 12-kDa human FK506 binding domain (FKBP12; GenBank AH002 818) that contains an F36V mutation, allowing dimerization of the caspase-9 and activation of the apoptotic pathway after exposure to the FK506 analog AP20187.18,21 The 3 genes (iCasp9, ΔCD34, and IL2 or IL15) were linked using 2A-like peptides derived from foot-and-mouth disease virus, to allow transcription and expression of one single mRNA molecule. The sequences of the 2A-like peptides were pSTA1-TaV RAEGRGSLLTCGDVEENPGP and pSTA1-ERAV QCTNYALLKLAGDVESNPGP.22,23 The entire cassette was cloned into the SFG retroviral vector and schema of SFG.iCasp-9.2A.ΔCD34.2A.IL-2 (iC.ΔCD34/IL-2v), SFG.iCasp-9.2A.ΔCD34.2A.IL-15 (iC.ΔCD34/IL-15v), and SFG.ΔCD34 (ΔCD34v) are illustrated in Figure 1A. These vectors were used for all the in vitro and in vivo experiments. The vector encoding the fusion protein eGFP-Fireflyluciferase (eGFP-FFLuc) was previously described.24 The retroviral supernatant was prepared as previously described.25 Briefly, 293T cells were cotransfected with 3 plasmids (the retroviral construct, Peg-Pam-e encoding for gag-pol, and DRF encoding for the RD114 envelop),26 using the Fugene6 transfection reagent (Roche, Indianapolis, IN), and supernatants were collected 48 and 72 hours later.
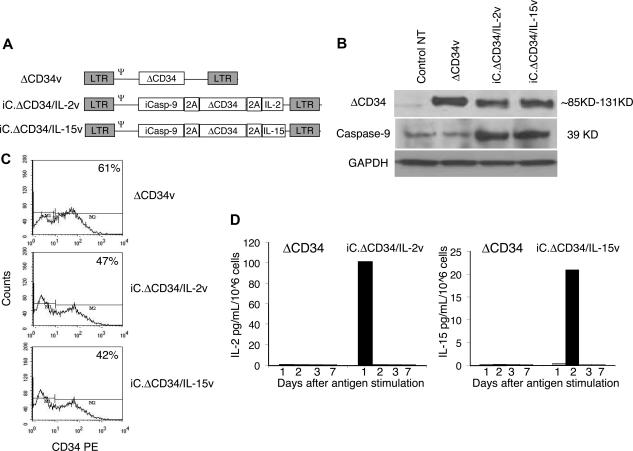
Figure 1.
Construction and functionality of the retroviral vectors. Panel A is the schema of the retroviral vectors used to transduce EBV-CTLs. Panel B is a Western blot analysis showing the expression of ΔCD34 (top panel) and caspase-9 (middle panel) in COS-7 cells transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v or ΔCD34v vectors. The lower gel shows the membrane reprobed with anti-GAPDH antibody. Panel C shows the transduction efficiency of EBV-CTLs measured as expression of a truncated form of CD34 (ΔCD34) on the cell surface by FACS analysis. Plots from a representative experiment are shown. Panel D illustrates the kinetics of cytokine release by EBV-CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v or ΔCD34v vectors and stimulated with EBV-LCLs. Cytokines were detected in the culture supernatant at the indicated time after EBV-LCL stimulation and measured by specific ELISAs.
Generation and transduction of EBV-CTLs
EBV-CTLs were prepared by stimulating peripheral blood mononuclear cells (PBMCs) with gamma-irradiated (40 Gy) autologous EBV-transformed lymphoblastoid cell lines (LCLs) on day 0 and + 9, then weekly thereafter. Recombinant human interleukin-2 (rhIL-2) (50 U/mL) (Proleukin; Chiron, Emeryville, CA) was added twice a week from day 14 as previously described.27 For transduction, EBV-CTLs obtained after at least 3 stimulations were plated at 0.5 × 106 cells/well in 24-well plates precoated with recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Shuzo, Otsu, Japan) and incubated with the retroviral supernatant.25 Three days after transduction, EBV-CTLs were collected and then stimulated weekly with autologous LCLs, with or without the addition of exogenous cytokines rhIL-2 (50 U/mL) or recombinant human IL-15 (rhIL-15) (10 ng/mL) (R&D Systems, Minneapolis, MN). The release of IL-2 and IL-15 by control and transgenic CTLs was measured in the culture supernatant by specific ELISAs from R&D Systems.
Immunophenotyping
Cells were stained with phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)–, or PerCP-conjugated monoclonal antibodies (MAbs). We used CD3, CD4, CD8, CD56, CD34, CD45RA, CD45RO, and CD62L from Becton Dickinson (Mountain View, CA) and MAbs specific for the TCR-Vβ repertoire (IOTest βMark kit; Immunotech, Emeryville, CA). Tetramers targeting known major histocompatibility complex (MHC) class I epitopes of EBV-related antigens also were used.25,28 The induction of apoptosis of EBV-CTLs was evaluated using the Annexin-V/7-AAD staining (Becton Dickinson). In some experiments Annexin-V+ CTLs were selected using Annexin-V-FITC antibody and anti-FITC magnetic beads (Miltenyi). Cells were analyzed by a FACScan (Becton Dickinson) equipped with the filter set for triple fluorescence signals.
Activation of the suicide gene
The chemical inducer of dimerization (CID) (AP20187; ARIAD Pharmaceuticals, Cambridge, MA) was kindly provided by Dr Spencer (Baylor College of Medicine) and added at the indicated concentrations to EBV-CTLs transduced either with ΔCD34v, or iC.ΔCD34/IL-2v, or iC.ΔCD34/IL-15v vectors. The elimination of transgenic cells co-expressing the iCasp-9 suicide gene was evaluated 24 to 48 hours later by FACS analysis, enumerating the percentage of ΔCD34 + cells in the culture. In parallel, we measured IL-2 and IL-15 cytokines in the culture supernatant using specific enzyme-linked immunosorbent assays (ELISAs) (R&D Systems). For long-term experiments, IL-2 and IL-15 transgenic CTLs were selected with magnetic beads (Miltenyi) based on their expression of ΔCD34, exposed to a single dose of CID (50 nM), and then maintained in culture by weekly stimulation with autologous LCLs, without addition of exogenous cytokines and without further addition of the CID. Viable cells were enumerated each week using trypan blue exclusion. Control CTLs were maintained in culture without exposure to CID.
Chromium release assay
We evaluated the cytotoxic activity of EBV-CTLs by using a standard 4-hour 51Cr release assay, as previously described.29 As target cells we used autologous LCLs, HLA class I and II mismatched LCLs, as well as the HSB-2 and K562 cell lines that measure lymphokine-activated and natural killer activity, respectively. Target cells incubated in media alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts - spontaneous counts)/(maximum counts - spontaneous counts)] × 100.
Enzyme-linked immunospot assay
The interferon γ (IFNγ) enzyme-linked immunospot (ELISPOT) assay was performed as previously described.25,28,30 We plated EBV-CTLs in triplicate, serially diluted from 105 to 104 cells/well and stimulated with 100 μL of autologous, irradiated LCLs (105 cells) or EBV-derived peptides (5 μM). Negative controls included EBV-CTLs alone and EBV-CTLs loaded with irrelevant peptides.
Western blot analysis
Cell lysates were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Caspase-9 and CD34 proteins were detected by immunoblot using specific monoclonal Abs (Abcam Inc, Cambridge, MA). Immunoblots were developed using enhanced chemiluminescence detection reagents (Amersham Biosciences, Freiburg, Germany). Membranes were reprobed using the monoclonal anti-GAPDH Ab (Santa Cruz Biotechnologies, Santa Cruz, CA).
In vivo study using a xenogenic SCID mouse model
To assess the expansion, persistence, and antitumor effect of transgenic EBV-CTLs in vivo, we used a SCID mouse model and an in vivo imaging system. CB17 SCID mice 8 to 10 weeks old were purchased from Harland Sprague Dawley, Indianapolis, IN. Mouse experiments were performed in accordance with Baylor College of Medicine Animal Husbandry guidelines.
In vivo expansion and antitumor effects of transgenic CTLs
To evaluate the in vivo expansion of transgenic CTLs, EBV-CTLs transduced either with iC.ΔCD34/IL-2v, iC.ΔCD34/IL-15v, or ΔCD34v control vector were transduced a second time with the vector encoding the eGFP-FFLuc gene.24,31 A total of 4 different EBV-CTL lines were tested in vivo. Briefly, SCID mice were sublethally irradiated (250 rad) and injected subcutaneously with 107 LCLs suspended in Matrigel (Becton Dickinson). Between 15 and 20 days later, when the tumor was palpable (0.5–0.8 cm in diameter), 107 autologous CTLs were injected intravenously. No exogenous cytokines were administered. For the in vivo imaging of EBV-CTLs expressing eGFP-FFLuc, mice were injected intraperitoneally with D-luciferin (150 mg/kg) and analyzed using the Xenogen-IVIS Imaging System, as previously described.24 Briefly, a constant region-of-interest (ROI) was drawn over the tumor region and the intensity of the signal measured as total photon/sec/cm2/sr (p/s/cm2/sr).32 Mice were euthanized if their tumor was more than 1.2 cm in diameter. Mice with smaller tumors or complete tumor regression were followed until day 50 after CTL infusion and then euthanized to analyze the tumors after autopsy. A group of control mice engrafted with LCLs received unmanipulated EBV-CTLs and rhIL-2 intraperitoneally 1000 U/mL every 2 days.33
In vivo validation of the iCasp-9 suicide gene
To evaluate the functionality of the suicide gene, mice bearing LCLs and receiving EBV-CTLs transduced either with iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vector and labeled with the eGFP-FFluc gene were treated with CID (50 μg) intraperitoneally 2 to 3 doses every other day. CID treatment was initiated when the bioluminescent signal was exponentially increasing, indicating active expansion of the transgenic cells. Mice were then imaged as described above.
Statistical analysis
All in vitro data are presented as mean plus or minus 1 SD. Student t test was used to determine the statistical significance of differences between samples, and P less than .05 was accepted as indicating a significant difference. For the bioluminescent experiments, intensity signals were log-transformed and summarized using mean plus or minus SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were calculated and compared using the Wilcoxon signed-rank test. Tumor-free survival was analyzed by Kaplan-Meier analysis (using SPSS software, SPSS, Chicago, IL), and the statistical significance of observed differences was assessed by log-rank and Breslow testing.
Results
Transgenic expression of IL-2 and IL-15 sustains the expansion of EBV-CTLs
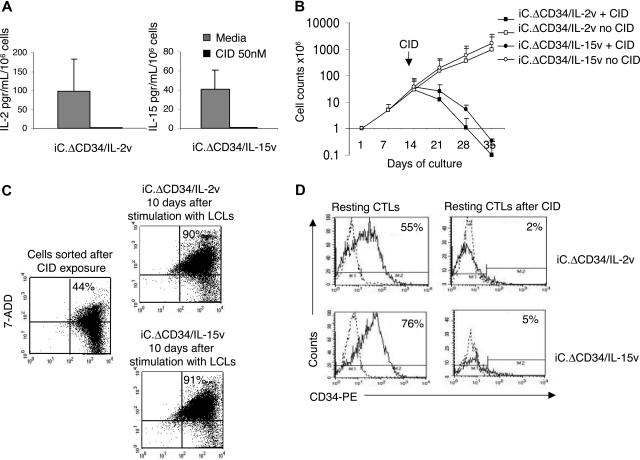
Figure 1A shows the construction schema for the vectors SFG.iCasp-9.2A.ΔCD34.2A.IL-2 (iC.ΔCD34/IL-2v), SFG.iCasp-9.2A.ΔCD34.2A.IL-15 (iC.ΔCD34/IL-15v) and SFG.ΔCD34 (ΔCD34v) used to transduce EBV-CTL lines. To validate expression from these vectors, we performed a Western blot analysis using COS-7–transduced cell lines. Figure 1B illustrates that CD34 and Caspase-9 proteins were expressed as single proteins in cells transduced either with iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors, indicating efficient cleavage mediated by the 2A-like sequence. We were not able to determine the presence of the cytokines IL-2 and IL-15 by Western blot, likely because these cytokines were rapidly secreted. However, these cytokines were detectable in the culture supernatants of transduced but not control COS-7 cells (data not shown). We obtained EBV-CTLs after 3 stimulations of T cells with autologous LCLs from 5 healthy EBV-seropositive donors27 and transduced them either with iC.ΔCD34/IL-2v, or iC.ΔCD34/IL-15v, or ΔCD34v vectors. We determined the transduction efficiency of EBV-CTLs by surface detection of the CD34 molecule and obtained values of 33% (± 15%), 37% (± 16%), and 56% (± 20%) for the iC.ΔCD34/IL-2v, iC.ΔCD34/IL-15v, and ΔCD34v vector, respectively (Figure 1C). To confirm the functionality of the constructs, we used specific ELISAs to measure the amount of IL-15 and IL-2 cytokines released in the supernatants of EBV-CTL lines stimulated with autologous EBV-LCLs. IL-15 was undetectable in control EBV-CTL supernatants, but was 41 pg/mL/106 cells (range, 21 to 72) in supernatants from EBV-CTLs transduced with iC.ΔCD34/IL-15v, 48 to 72 hours after the first stimulation with autologous LCLs. The production of IL-15 by transgenic CTLs remained detectable, although at lower levels, after antigen stimulation in supernatants of transgenic CTLs maintained in culture for more than 4 weeks (data not shown). The maximum concentration of IL-2 was detected 24 hours after the first stimulation with LCLs. It was 40 pg/mL/106 cells (range, 0 to 101) in control EBV-CTLs and 108 pg/mL/106 cells (range, 24 to 264) (P = .08) in EBV-CTLs transduced with the iC.ΔCD34/IL-2v. Although the increased production of IL-2 by iC.DCD34/IL-2v–transduced CTLs did not reach statistical significance compared with control CTLs after the first LCL stimulation, IL-2 remained detectable (7-10 pg/mL) in the supernatant for up to 5 rounds of restimulation with antigen. In contrast, IL-2 was always undetectable in control CTLs immediately after the first stimulation with EBV-LCLs, and these cells did not significantly expand. To confirm that IL-2 produced by IL-2 transgenic CTLs was responsible for the growth of CTL lines transduced with iC.ΔCD34/IL-2v vector, we used blocking antibodies to the IL-2 cytokine or to its high-affinity receptor (CD25) (R&D Systems). In both cases, we observed significant inhibition of the proliferation of IL-2 transgenic CTLs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
We also evaluated the kinetics of IL-2 and IL-15 cytokine production by CTLs transduced either with iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v or ΔCD34v vectors. Figure 1D shows that cytokine production was antigen dependent and that both IL-2 and IL-15 secretion from CTLs became undetectable 6 to 7 days after antigen stimulation.
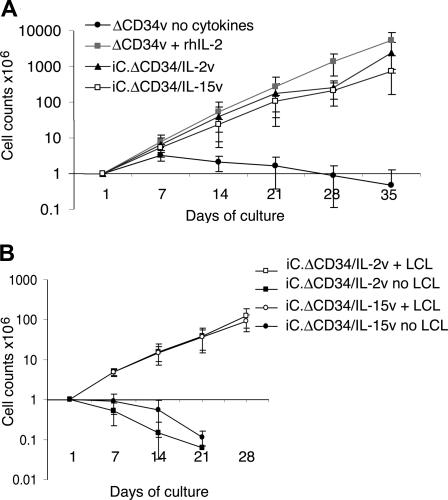
We next evaluated whether the quantity of cytokine production was sufficient to sustain the expansion of transgenic EBV-CTLs in response to specific antigenic stimulation. After transduction, EBV-CTLs were maintained in culture and stimulated once a week with autologous LCLs (E/T ratio of 1:1) without addition of exogenous cytokines. In parallel, control EBV-CTLs transduced with ΔCD34v vector alone were maintained in culture with exogenous rhIL-2 (50 U/mL) or rhIL-15 (10 ng/mL). As shown in Figure 2A, a significant expansion of IL-2 transgenic CTLs (1838-fold expansion; range, 289-5044) and IL-15 transgenic CTLs (1156-fold expansion; range, 511-3069) was observed after 35 days of culture. As expected, control EBV-CTLs transduced with ΔCD34v vector also expanded when stimulated with LCLs, but only in the presence of exogenous rhIL-2 (3576-fold expansion; range, 915-5000) or rhIL-15 (1389-fold expansion; range, 335-2838). In the absence of such exogenous cytokines, LCL-stimulated ΔCD34v CTLs did not significantly expand (< 2-fold). Similar results were observed using CD4+ EBV-CTL lines (Figure S2). We found that the continued proliferation of transgenic EBV-CTLs remained strictly antigen dependent, as a progressive reduction in the proportion of stimulator cells (CTL:LCL ratio from 4:1 to 10:1) resulted in a corresponding reduction in the CTL's expansion rate (data not shown). Moreover, both IL-2 and IL-15 transgenic EBV-CTLs maintained in culture without antigen stimulation did not significantly expand and died within 2 to 3 weeks (Figure 2B).
Figure 2.
IL-2 and IL-15 transgenic CTLs expand in response to antigen stimulation. EBV-CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v or ΔCD34v vectors were maintained in culture and stimulated once a week with autologous LCLs (E/T ratio 1:1) without addition of exogenous cytokines. Control EBV-CTLs transduced with the ΔCD34v vector were maintained in culture by adding rhIL-2 (50 U/mL). Data represent the mean plus or minus SD of cell expansion of 5 donors (panel A). IL-2 or IL-15 transgenic EBV-CTLs did not significantly expand when they were maintained in culture without specific antigen stimulation. Data represent the mean plus or minus SD of cell expansion of 3 donors (panel B).
IL-2 and IL-15 transgenic CTLs retain their antigen specificity
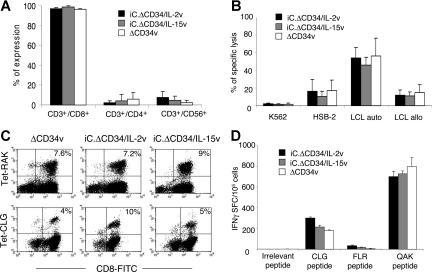
To confirm that the genetic manipulation of EBV-CTLs did not modify their antigen specificity, we monitored their phenotype, polyclonality, and antigen repertoire over 5 to 6 weeks of culture. The phenotypic profile of CTLs was not altered by cytokine transduction, as most EBV-CTLs remained CD3+/CD8+ (96% ± 1%, 97% ± 2%, and 98% ± 2% for ΔCD34v, iC.ΔCD34/IL-2v and iC.ΔCD34/IL-15v vectors), while less than 5% were CD3+/CD4+. In all cases, fewer than 15% of cells were CD3+/CD56+ (Figure 3A). EBV-CTL lines growing in exogenous rhIL-2 are mainly effector cells with a proportion of effector memory cells (CD45RA−, CD45RO+, CD62L+) ranging from 2% to 15%.25 This pattern was similarly maintained in CTLs expanded with exogenous rhIL-15 or in CTLs transgenic for IL-2 or IL-15. In addition, neither IL-2 nor IL-15 transgenes modified the antigen specificity of the EBV-CTLs. Hence, cytotoxic activity, measured by 51Cr release assay, remained specific for autologous LCLs, and the percentage of autologous LCLs lysed by the control EBV-CTLs was 56% (± 20%) at an E/T ratio of 20:1, versus 54% (± 12%) for IL-2 and 46% (± 9%) for IL-15 transgenic cells (Figure 3B). Killing of allogeneic LCLs was significantly lower in all cases, confirming retained MHC restriction (Figure 3B). No significant reactivity (< 10%) was observed against the K562 cell lines (Figures 3B, S3).
Figure 3.
IL-2 and IL-15 transgenic CTLs maintain their antigen specificity. EBV-CTLs transduced with either ΔCD34v or iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors were analyzed for their phenotype and antigen specificity. Panel A illustrates the phenotypic analysis of transduced EBV-CTLs. Data represent the mean (± SD) of 5 donors. Panel B illustrates the result of a standard 51Cr release assay in which killing by CTLs of autologous LCLs, allogeneic LCLs, HSB-2 and K562 cell lines was tested at an E/T ratio of 20:1. Data represent the mean (± SD) of 5 donors. Panel C illustrates a representative staining of EBV-CTLs using tetramers targeting the HLA-B8 BZLF1 peptide RAKFKQLL and the HLA-A2 LMP-2 peptide CLGGLLTMV. Panel D illustrates the IFN-γ ELIspot assay of EBV-CTLs tested against the EBV-peptides HLA-A2 LMP-2 CLGGLLTMV, HLA-B8 EBNA3A FLRGRAYGL and HLA-B8 BZLF1 QAKWRLQTL. Data represent the mean (± SD) of 3 donors.
To further demonstrate that EBV-CTLs maintained the pattern of antigen response after transduction, we evaluated the frequency with which each EBV-CTL population recognized HLA class I–restricted EBV peptides, using specific peptide HLA tetramers and flow cytometric analysis to measure binding and IFNγ ELIspots to measure responsiveness. As shown in Figure 3C,D and Table 1, the frequency of EBV-specific CTLs in control and transgenic CTLs was retained. Although the distribution of antigen specificity and Vβ T-cell receptor (αβTCR) drifted over time, there was continued polyclonality of the transgenic EBV-CTLs, with no evidence for progressive clonal outgrowth compared with control CTLs growing with exogenous rhIL-2 (Table S1).
Table 1.
Tetramer analysis of EBV-CTL lines
| EBV-CTL lines (HLA type) |
B8 RAK | B8 QAK | A2 CLG |
|---|---|---|---|
| Transgene expression | |||
| No. 1 (HLA-A2,24;B8,65) | |||
| ΔCD34 | 8.9% | 4.3% | NT |
| iC.ΔCD34/IL-2 | 9.6% | 4.7% | NT |
| iC.ΔCD34/IL-15 | 12.2% | 1.8% | NT |
| No. 2 (HLA-A1,24;B8,18) | |||
| ΔCD34 | 0.7% | 9.3% | NT |
| iC.ΔCD34/IL-2 | 1.5% | 10.4% | NT |
| iC.ΔCD34/IL-15 | 0.7% | 31.4% | NT |
| No. 3 (HLA-A2;B8,57) | |||
| ΔCD34 | 2.3% | 26% | 9.3% |
| iC.ΔCD34/IL-2 | 2% | 3.6% | 13% |
| iC.ΔCD34/IL-15 | 1.4% | 1.3% | 6.2% |
| No. 4 (HLA-A1,2;B7,8) | |||
| ΔCD34 | 5.7% | NT | 0.4% |
| iC.ΔCD34/IL-2 | 5.8% | NT | 0.3% |
| iC.ΔCD34/IL-15 | 3.6% | NT | 0.4% |
The following HLA class I–restricted tetramers were used: HLA-A2 LMP2 epitope CLGGLLTML (CLG), HLA-B8 BZLF1 epitope RAKFQLL (RAK), HLA-B8 EBNA3A QAKWRLQTL (QAK).
ΔCD34 indicates CTLs transduced with the ΔCD34 control vector; iC.ΔCD34/IL-2v, CTLs transduced with the iC.ΔCD34/IL-2v; iC.ΔCD34/IL-15v, CTLs transduced with the iC.ΔCD34/IL-15v; and NT, not tested.
Expression of transgenic cytokines improves in vivo expansion and antitumor activity of EBV-specific CTLs
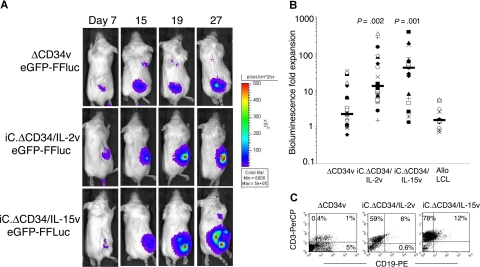
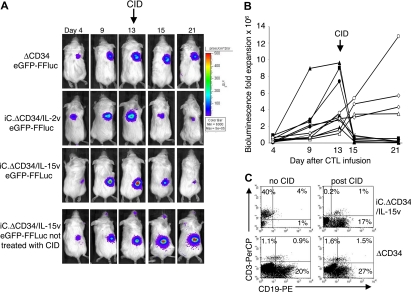
To assess the in vivo functionality of transgenic CTLs, we used a SCID mouse tumor model, in which the animals were engrafted subcutaneously with EBV-LCLs and then infused intravenously with autologous EBV-CTLs.33 Transduced EBV-CTLs obtained from 4 healthy donors were selected with anti-CD34 microbeads and then transduced with the vector encoding eGFP-FFLuc.24 After the second transduction, the percentage of GFP+ EBV-CTLs ranged from 43% to 64% for ΔCD34v, from 34% to 46% for iC.ΔCD34/IL-2v, and from 16% to 46% for iC.ΔCD34/IL-15v. SCID mice were engrafted with autologous LCLs (107 cells), and when the tumor was palpable (0.5 to 0.8 cm in diameter), 107 EBV-CTLs were injected intravenously. No exogenous cytokines were administered. Localization and expansion of the CTLs in the tumor area were measured using the Xenogen-IVIS Imaging System. As illustrated in Figure 4A,B, which summarize results from 20 mice per group, the EBV-CTLs homed to the tumor and progressively expanded. Maximum bioluminescence photon emission occurred 2 to 4 weeks after CTL infusion, and the median increase was 2-fold (range, 0.5 to 45) for control ΔCD34v, 13-fold (range, 1.2 to 587) for iC.ΔCD34/IL-2v (P = .002), and 33-fold (range, 1.5 to 97) for iC.ΔCD34/IL-15v (P < .001).
Figure 4.
In vivo expansion of IL-2 and IL-15 transgenic CTLs. SCID mice engrafted with LCLs were injected with either EBV-CTLs control (ΔCD34v) or EBV-CTLs transgenic for IL-2 (iC.ΔCD34/IL-2v) or IL-15 (iC.ΔCD34/IL-15v) (107 cells). To track their homing and in vivo expansion, CTLs were transduced with the vector encoding eGFP-FFLuc. CTL localization and expansion were monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Mice did not receive exogenous cytokines after CTL transfer. Panel A shows images of representative mice. The signal intensity measured as photon/sec/cm2/sr (p/s/cm2/sr) was increased in mice receiving CTLs transgenic for IL-2 or IL-15 compared with control cells (ΔCD34v). Panel B illustrates the maximum increase in bioluminescence obtained in 20 mice per group. The expansion of iC.ΔCD34/IL-15v CTLs and iC.ΔCD34/IL-2v CTLs was statistically significant compared with control ΔCD34v (P < .001 and P < .002, respectively). IL-2 and IL-15 transgenic CTLs did not significantly expand in response to allogeneic EBV-LCLs. (C) To evaluate whether the increase in bioluminescence signal corresponded to an increased number of CTLs infiltrating the tumor, mice were euthanized and T-cell infiltration in the bioptic samples was measured using antihuman CD3 staining and FACS analysis (ΔCD34v = 3.8 × 105 p/s/cm2/sr; iC.ΔCD34/IL-2v = 3 × 106 p/s/cm2/sr; iC.ΔCD34/IL-15v = 3.7 × 106 p/s/cm2/sr).
The increase of bioluminescence from IL-2 and IL-15 transgenic CTLs remained antigen dependent and MHC restricted in vivo: when the mice were engrafted with allogeneic EBV-LCLs, transgenic CTLs homed to the tumor but, unlike with autologous EBV-LCLs, did not expand significantly (Figure 4B). The increase in the bioluminescence signal for the cytokine-transgenic CTLs corresponded to an actual increase in number of CTLs accumulated within the tumor and not just to an increase in bioluminescence due to enhanced expression by the same number of cells. Figure 4C shows the increased CTL numbers in tumors excised from animals in the high (cytokine-transduced) versus the low (control) bioluminescence groups.
The increased expansion in vivo of cytokine transgenic CTLs was associated with an enhanced antitumor effect, as in both the IL-2 and the IL-15 transgenic CTL groups, 47% to 53% of the mice were tumor free 4 to 6 weeks after adoptive T-cell transfer, compared with none of the mice receiving control CTLs (P = .001 for iC.ΔCD34/IL-2v CTLs and P < .001 for iC.ΔCD34/IL-15v CTLs) (Table 2 and Figure S4). Tumor regression was observed in mice treated with transgenic IL-2 and IL-15 from 3 of the 4 EBV-CTL lines used (25% for donor no. 1, 75% for donor no. 2, 64% for donor no. 3, and 0% for donor no. 4). These results also compared favorably to mice receiving unmanipulated CTLs and exogenous rhIL-2 in which we observed a 17% rate of complete tumor regression33 (P = .045 for iC.ΔCD34/IL-2v CTLs and P = .019 for iC.ΔCD34/IL-15v CTLs, respectively) (Table 2).
Table 2.
Antitumor effect of EBV-CTL adoptive transfer
| Mice | EBV-CTLs |
EBV-CTLs |
EBV-CTLs |
EBV-CTLs |
|---|---|---|---|---|
| ΔCD34v | + rhIL-2 | iC.ΔCD34/IL-2v | iC.ΔCD34/IL-15v | |
| Inoculated with EBV-LCLs | 17 | 24 | 19 | 17 |
| Tumor free 4 to 6 weeks after CTL transfer | 0 | 4 (17%) | 9 (47%)* | 9 (53%)† |
ΔCD34v indicates CTLs transduced with the ΔCD34 control vector; iC.ΔCD34/IL-2v, CTLs transduced with the vector encoding for IL-2; iC.ΔCD34/IL-15v, CTLs transduced with the vector encoding for IL-15v. Mice injected with these CTLs did not receive exogenous cytokines. EBV-CTLs indicates CTLs non transduced. Mice infused with control EBV-CTLs received exogenous rhIL-2 (1000 U/mL) intraperitoneally every 2 days.
P = .001.
P < .001 when compared to control EBV-CTLs ΔCD34v by Fisher exact test.
Activation of the iCasp-9 suicide gene eliminates transgenic EBV-CTLs ex vivo and in vivo
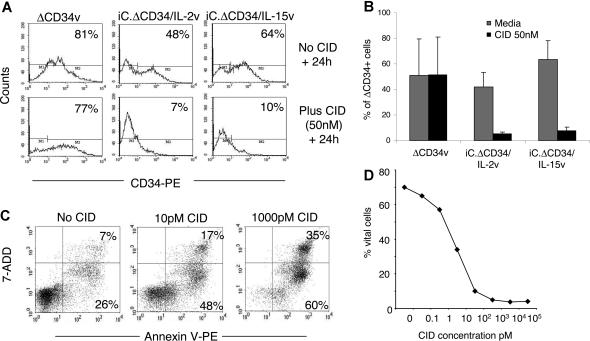
For clinical applications, constitutive expression of transgenic cytokines would likely raise concerns about autonomous and uncontrolled growth of CTLs.16,17 We therefore incorporated the iCasp-9 suicide gene in our cytokine encoding retroviral vectors.18 This proapoptotic gene product is activated after exposure to a small CID (AP20187), which is an analog of FK506. Addition of CID (50 nM) to cultures of EBV-CTLs transduced either with iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors led to a significant reduction in the percentage of ΔCD34+ cells (from 42% ± 11% to 5% ± 1%, P = .002 for iC.ΔCD34/IL-2v and from 63% ± 15% to 8% ± 3%, P < .001 for iC.ΔCD34/IL-15v) within 24 to 48 hours (Figure 5A,B). In contrast, the percentage of ΔCD34+ cells did not change when the drug was added to EBV-CTLs transduced with the ΔCD34v control vector, which lacks the iCasp-9 gene. Elimination of the transgenic cells in vitro was dose dependent (Figure 5C,D) and was mediated by induction of apoptosis as assessed by Annexin-V/7-AAD staining. Although up to 10% of ΔCD34dim cells persisted 24 to 48 hours after incubation with CID, the near complete elimination of ΔCD34bright cells meant that the (linked) production of IL-2 and IL-15 cytokines fell to unmeasurable levels (Figure 6A). To further demonstrate elimination of the transgenic cells, we evaluated whether exposure to CID terminated the growth of IL-2 or IL-15 transgenic CTLs in long-term culture experiments. Transgenic CTLs were selected based on the expression of ΔCD34 (supplemental Figure 5) and incubated with CID (50 nM) for 24 to 48 hours. CTLs were then collected, washed, and restimulated once a week with autologous LCLs for a total of 3 stimulations without any addition of CID. As shown in Figure 6B, a single dose of drug was sufficient to abrogate the long-term growth of IL-2 or IL-15 transgenic CTLs, and the cells progressively died. To evaluate whether the fraction of apoptotic cells (Annexin-V+/7-AAD−) detected 24 to 48 hours after CID exposure could recover and become a viable cell population (Annexin-V−/7-AAD−) after drug withdrawal, we sorted Annexin-V+ cells using magnetic beads and maintained them in culture with or without stimulation with autologous LCLs. As shown in Figure 6C, Annexin-V+ cells were not restored to an Annexin-negative population even after antigenic stimulation, and instead they invariably progressed to late apoptosis/necrosis (Annexin-V+/7-AAD+). We also evaluated whether the CID could induce apoptosis in resting transgenic CTLs. IL-2 and IL-15-transgenic CTLs sorted for CD34 expression and maintained in culture for 8 to 9 days after the last stimulation were incubated with CID (50 nM). As shown in Figure 6D, the drug continued to significantly deplete the transduced cells within 24 to 48 hours.
Figure 5.
Activation of the iCasp-9 suicide gene significantly eliminates IL-2 and IL-15 transgenic CTLs. EBV-CTLs transduced with either ΔCD34v or iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors were plated at 106 cells/well and incubated with or without CID AP20187 at 50 nM. Twenty-four hours later cells were collected, stained with CD34-PE antibody, and the percentage of residual transgenic CTLs was evaluated by FACS analysis. Significant reduction of CD34+ cells after incubation with CID was obtained only for EBV-CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15 vectors incorporating the iCasp-9 gene. Panel A illustrates ΔCD34 expression in a representative experiment. Panel B summarizes the effects of the CID on 4 different EBV-CTL lines transduced with either iC.ΔCD34/IL-2v, iC.ΔCD34/IL-15v, or ΔCD34v vectors. The y axis represents the mean (± SD) for CD34+ CTLs in the CTL lines before or after incubation with CID. The percentage of CD34+ cells remained unchanged in control CTLs transduced with the ΔCD34v vector lacking the suicide gene. In contrast, a significant reduction in the percentage of CD34+ CTLs was observed for CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors. Panel C shows that the elimination of transgenic CTLs was mediated by induction of apoptosis as assessed by Annexin-V/7-AAD staining. Panel D shows that the induction of apoptosis/necrosis by CID was dose dependent. CTLs transgenic for IL-2 or IL-15 and selected using CD34 magnetic beads were incubated with different doses of CID, and then 24 hours later the induction of apoptosis/necrosis was evaluated by staining with Annexin-V/7-ADD and FACS analysis.
Figure 6.
Activation of the iCasp-9 suicide gene abrogates cytokine production and long-term expansion of IL-2 and IL-15 transgenic CTLs. EBV-CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors were plated at 106 cells/well and incubated with or without the CID AP20187 (50 nM). Panel A illustrates the production of transgenic cytokines IL-2 or IL-15 by iC.ΔCD34/IL-2v and iC.ΔCD34/IL-15v EBV-CTLs before and after exposure to the CID. Neither IL-2 nor IL-15 cytokines could be detected in the supernatants from CTLs treated with the CID. Data represent the mean (± SD) of 4 experiments. Panel B illustrates the long-term expansion of EBV-CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vectors and stimulated once a week with autologous LCLs. Viable cells were counted by trypan blue exclusion once a week before EBV-LCL restimulation. Exposure to a single dose of CID (50 nM) ablated CTL expansion, while nonexposed CTLs continued to expand. Data represent mean (± SD) of 4 experiments. Panel C illustrates a representative experiment in which IL-2 and IL-15 transgenic CTLs obtained 24 hours after CID exposure were sorted based on the expression of Annexin-V. These CTLs were then cultured without any further addition of CID and stimulated with EBV-LCLs. Annexin-V/7ADD staining showed that these cells progressed to a late stage of apoptosis/necrosis (Annexin-V+/7ADD+) by days 7 to 9. Panel D shows that CID induced elimination of CD34+ cells for IL-2 or IL-15 transgenic CTLs even in their resting phase, 7 days after the last antigen stimulation.
The iCasp-9 system also was functional in vivo. iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v–transduced EBV-CTLs were selected for ΔCD34 expression and then transduced with the eGFP-FFLuc gene, as described above. Ten million EBV-CTLs were injected intravenously in tumor-bearing SCID mice. Once CTL expansion was detectable at the tumor site (assessed by progressively rising photon emission), the animals were injected intraperitoneally with CID. As illustrated in Figure 7A,B, photon emission at the time of CTL localization at the tumor site ranged from 5 × 104 to 105 p/s/cm2/sr, increasing to 1.8 × 106 to 1.5 × 107 p/s/cm2/sr (P < .01) by days 10 to 15. Mice were then treated with CID (50 μg) and the bioluminescence fell by more than 1 log (6.3 × 104 to 5.2 × 105 p/s/cm2/sr) within 24 to 72 hours, returning to the pre-expansion level (P = .1), compared with the bioluminescence at the time of CTL localization. In contrast, the bioluminescence increased over time in mice infused with IL-2 or IL-15 transgenic CTLs without the CID (Figure 7A,B). As shown in Figure 7C, the reduction of the bioluminescence after CID administration corresponded to a significant decrease in CTLs infiltrating the tumor, assessed by FACS analysis of biopsied tumors.
Figure 7.
Activation of the iCasp-9 suicide gene eliminates the IL-2 and IL-15 transgenic CTLs in vivo. SCID mice engrafted subcutaneously with LCLs were injected intravenously with EBV-CTLs transduced with either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v vector, sorted for ΔCD34 expression, and transduced with eGFP-FFLuc vector. When the CTLs were expanding, mice were treated with 2 to 3 doses of the CID AP20187 (50 μg) intraperitoneally 2 days apart. The persistence of the transgenic cells was monitored in vivo using the bioluminescence system. Panel A illustrates in a representative experiment the reduction of the bioluminescence after CID administration. Bioluminescence was significantly reduced in mice receiving IL-2 or IL-15 transgenic CTLs after treatment with CID. In contrast, the signal was not diminished in mice receiving control ΔCD34+ CTLs lacking the expression of the suicide gene. The bioluminescence continued to increase in mice receiving IL-15 transgenic CTLs nontreated with CID. Panel B shows the kinetics of bioluminescence in 7 mice (closed symbols) before and after treatment with CID. In mice receiving EBV-CTLs expressing either iC.ΔCD34/IL-2v or iC.ΔCD34/IL-15v followed by the CID greater than 1 log reduction in bioluminescence was observed. In contrast, bioluminescence continued to increase in mice nontreated with CID (4 representative mice, open symbols). (C) Mice showing > 106 photons were euthanized to evaluate the infiltrate of CTLs within the tumor by FACS analysis after staining with antihuman CD3 antibody (top left panel). Mice with similar level of bioluminescence signal were treated with CID and 24 to 72 hours later euthanized to evaluate the effective reduction of CTL infiltration by FACS analysis after staining with antihuman CD3 antibody (top right panel). CID did not reduce the number of CD3+ cells in mice receiving control CTLs transduced with ΔCD34v vector (bottom left and right).
Discussion
We have used retroviral mediated transgenic expression of IL-2 or IL-15 in tumor-specific CTLs to improve their expansion and efficacy. We also determined whether the inclusion of a suicide gene could remove the transgenic cells, to further increase safety should unwanted T-cell proliferation or autonomy occur. Using EBV as our model system, we found that IL-2 or IL-15 transgenic EBV-CTLs have enhanced expansion ex vivo and in vivo and retained their antigen specificity and effector function. Their improved survival and expansion were associated with increased antitumor activity in vivo. Importantly, we found that the growth of cytokine transgenic CTLs remained dependent on antigen stimulation and that the pharmacologic activation of a suicide gene iCasp-9 efficiently eliminated cytokine-producing CTLs.
Preclinical and clinical trials of adoptive transfer with antitumor CTLs have shown that the limited in vivo expansion of transferred CTLs is one of the barriers that needs to be overcome to improve the clinical outcome of this therapeutic approach.7,34 Transgenic expression of growth-promoting cytokines by the adoptively transferred CTLs represents a strategy to support their own expansion/persistence.13,14 In addition, the localized production of cytokines by transgenic cells might avoid the toxicity of systemic administration of recombinant cytokines and reduce the expansion of other cells, such as regulatory T cells that impair the antitumor immune response.7,9,11,12 However, before translating this genetic modification in a clinical application, several issues need to be addressed, such as confirming that progeny of genetically modified cells maintain antigen specificity and dependence, providing evidence that genetically modified CTLs possess enhanced antitumor effects, and ensuring that these cells remain safe.
Our model of EBV-tumor and EBV-CTLs demonstrates that retroviral gene transfer of IL-2 or IL-15 produces sufficient cytokines to sustain CTL expansion ex vivo and in vivo for more than 5 weeks, providing an improved antitumor effect. Importantly, the antigen specificity and dependence, and the MHC restriction of the CTL progeny, were maintained. Although extensive ex vivo T-cell culture can alter the antigenic and TCR repertoire of CTL lines, we found that IL-2 and IL-15 transgenic CTL lines retained broad reactivity to EBV epitopes and showed no progressive restriction of their antigen specificity or TCR repertoire compared with control CTL lines.
In a previously described immunocompetent mouse model, the presence of IL-15 resulted in the polarization to central memory phenotype of adoptively transferred CTLs and improved antitumor effects compared with IL-2.35 In our model, we did not find differences in antitumor effects between IL-2 or IL-15 transgenic EBV-CTLs or a polarization to central memory phenotype in IL-15 transgenic CTLs. Since the gene transfer was performed in CTL lines obtained after 2 to 3 stimulations ex vivo, in cells exposed to IL-2 for 10 to 15 days, it is possible that at the time of transduction, cells are already polarized to an effector memory phenotype and that the transgenic production of IL-15 cannot then affect this phenotype.
The use of cytokine-expressing CTLs in clinical adoptive transfer raises potential concerns, since leukemic transformation or immortalization of T cells has been reported in a mouse model engrafted with murine T-cell lines expressing IL-2,36 in IL-15 transgenic mice,37 and in a human T-cell clone.14 By contrast, our study of cytokine-expressing EBV-CTLs failed to show any evidence for progressive clonality or for the development of antigen-independent proliferation. In the absence of antigen, the transgenic CTLs did not expand and died within 2 to 3 weeks. Moreover, we found that the bioluminescence signal from transgenic CTLs progressively declined in vivo after elimination of the tumor. This is likely related to the fact that transgenic cytokine production depends on the activation of the retroviral LTR, which in turn is strictly dependent on the activation status of the T cells and is optimal only in the presence of adequate αβTCR stimulation.13,38 Moreover, the cells we transduce are mature effector T cells, which are likely less vulnerable to malignant transformation than the less-committed T progenitor cells or cell lines used in the earlier studies.36,37
Although our observations support the safety of transgenic expression of cytokines in antigen-specific CTLs, we cannot exclude rare oncogenic events associated with retroviral integration near promoters or genes involved with critical elements of growth and survival.14,16,17 We therefore incorporated a suicide gene based on the inducible caspase-9 molecule within our constructs. To generate these retroviral vectors encoding for 3 genes, we coexpressed iCasp-9, ΔCD34, and cytokine genes using 2A-like peptides,22,23 which use a ribosomal skip mechanism to allow translation of multiple genes encoded by one single mRNA.39 We found this approach for expressing multiple genes preferable to an internal ribosome entry site (IRES) or additional promoters, since these approaches were associated with suboptimal or unequal expression of one of the transgenes.23 Consistent co-expression of multiple transgenes is particularly important for our application, as the activation of the suicide gene needs to have its highest probability of success against those cells that produce the highest quantity of growth-promoting cytokines. While our data show that a fraction of transgenic CTLs (< 10%) did not undergo apoptosis/necrosis shortly after exposure to the dimerizer drug, these cells all were characterized by low expression of the ΔCD34 selectable marker and undetectable cytokine secretion. Hence, cytokine production falls to undetectable levels after suicide gene activation, and the increased proliferation following antigen stimulation is no longer observed.
The inclusion of the selectable marker within the retroviral cassette might be useful for the enrichment of transgenic CTLs for a clinical trial. We used a truncated form of human CD34 since CD34+ selection can be performed using clinical grade reagents. However, ΔCD34 can be easily substituted by other selectable markers, such as CD1940 in case it is found that CD34 expression impairs the trafficking in vivo of antigen-specific CTLs.
In conclusion, the data we report specifically support the use of transgenic cytokines to improve the expansion and antitumor effects of antigen specific CTLs. The incorporation of an effective suicide gene should further increase the safety of the approach and increase its potential clinical applicability.
Supplementary Material
Acknowledgments
This work was supported in part by the Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018) and MDACC SPORE in Head and Neck. B.S. is supported by National Institutes of Health grant no. 5R21AI65549. G.D. is supported by the Doris Duke Charitable Foundation/Clinical Scientist development award and by a Leukemia and Lymphoma Society Translational Research grant. J.V. and H.E.H. are supported by a Doris Duke Distinguished Clinical Scientist Award.
We express gratitude to Fernando Jimenez for FACS analysis, Jessie Wu for statistical analysis, and David Spencer for providing expertise with the suicide gene.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.Q. and J.V. contributed equally to the work; C.Q., J.V., B.S, G.G., and G.D. designed and performed the experiments; J.V., M.P., and G.D. designed and constructed the vectors; G.D., B.S., J.V., C.Q., and M.K.B. designed the research and analyzed the data; A.F. contributed to perform the in vivo experiments; H.E.H. and C.M.R. provided expertise in T-cell generation, analyzed the data, and reviewed the manuscript; G.D., B.S., J.V., C.Q., and M.K.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gianpietro Dotti, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, MC 3–3320, Houston, TX 77030; e-mail: gdotti@bcm.tmc.edu.
References
- 1.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roskrow MA, Suzuki N, Gan Y, et al. Epstein-Barr virus (EBV)–specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease. Blood. 1998;91:2925–2934. [PubMed] [Google Scholar]
- 4.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straathof KC, Bollard CM, Popat U, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 6.Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus–targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 7.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensinger SJ, Walsh PT, Zhang J, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biagi E, Rousseau R, Yvon E, et al. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2005;11:6916–6923. doi: 10.1158/1078-0432.CCR-05-0484. [DOI] [PubMed] [Google Scholar]
- 12.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu C, Hughes MS, Zheng Z, et al. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175:7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C, Jones SA, Cohen CJ, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109:5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 18.Straathof KC, Pule MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iuliucci JD, Oliver SD, Morley S, et al. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J Clin Pharmacol. 2001;41:870–879. doi: 10.1177/00912700122010771. [DOI] [PubMed] [Google Scholar]
- 20.Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem. 2000;275:30653–30659. doi: 10.1074/jbc.M002373200. [DOI] [PubMed] [Google Scholar]
- 21.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 22.Donnelly ML, Hughes LE, Luke G, et al. The “cleavage” activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring “2A-like” sequences. J Gen Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- 23.Szymczak AL, Workman CJ, Wang Y, et al. Correction of multi-gene deficiency in vivo using a single “self-cleaving” 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 24.Vera J, Savoldo B, Vigouroux S, et al. T-lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B-lymphocyte derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dotti G, Savoldo B, Pule M, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly PF, Carrington J, Nathwani A, Vanin EF. RD114-pseudotyped oncoretroviral vectors: biological and physical properties. Ann N Y Acad Sci. 2001;938:262–276. [PubMed] [Google Scholar]
- 27.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus–related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 28.Savoldo B, Goss JA, Hammer MM, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus–specific cytotoxic T lymphocytes (CTLs). Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus–induced lymphoma in allo-geneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 30.Savoldo B, Huls MH, Liu Z, et al. Autologous Epstein-Barr virus (EBV)–specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002;100:4059–4066. doi: 10.1182/blood-2002-01-0039. [DOI] [PubMed] [Google Scholar]
- 31.Savoldo B, Rooney CM, Di Stasi A, et al. Epstein Barr virus–specific cytotoxic T lymphocytes expressing the anti-CD30 (zeta) artificial chimeric T-cell receptor for immunotherapy of Hodgkin's disease. Blood. 2007 doi: 10.1182/blood-2006-11-059139. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YJ, Dubey P, Ray P, Gambhir SS, Witte ON. Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol Imaging Biol. 2004;6:331–340. doi: 10.1016/j.mibio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Lacerda JF, Ladanyi M, Louie DC, et al. Human Epstein-Barr virus (EBV)–specific cytotoxic T lymphocytes home preferentially to and induce selective regressions of autologous EBV-induced B cell lymphoproliferations in xenografted C. B-17 scid/scid mice. J Exp Med. 1996;183:1215–1228. doi: 10.1084/jem.183.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada G, Kitamura Y, Sonoda H, et al. Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J. 1987;6:2705–2709. doi: 10.1002/j.1460-2075.1987.tb02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fehniger TA, Suzuki K, VanDeusen JB, et al. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol Dis. 2001;27:223–230. doi: 10.1006/bcmd.2001.0379. [DOI] [PubMed] [Google Scholar]
- 38.Auten J, Agarwal M, Chen J, Sutton R, Plavec I. Effect of scaffold attachment region on transgene expression in retrovirus vector-transduced primary T cells and macrophages. Hum Gene Ther. 1999;10:1389–1399. doi: 10.1089/10430349950018058. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly ML, Luke G, Mehrotra A, et al. Analysis of the aphthovirus 2A/2B polyprotein “cleavage” mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal “skip.”. J Gen Virol. 2001;82:1013–1025. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- 40.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.