Abstract
Graft-versus-host disease (GVHD) continues to be a serious complication that limits the success of allogeneic bone marrow transplantation (BMT). Using IL-7–deficient murine models, we have previously shown that IL-7 is necessary for the pathogenesis of GVHD. In the present study, we determined whether GVHD could be prevented by antibody-mediated blockade of IL-7 receptor α (IL-7Rα) signaling. C57/BL6 (H2Kb) recipient mice were lethally irradiated and underwent cotransplantation with T-cell–depleted (TCD) BM and lymph node (LN) cells from allogeneic BALB/c (H2Kd) donor mice. Following transplantation, the allogeneic BMT recipients were injected weekly with either anti–IL-7Rα antibody (100 μg per mouse per week) or PBS for 4 weeks. Anti–IL-7Rα antibody treatment significantly decreased GVHD-related morbidity and mortality compared with placebo (30% to 80%). IL-7Rα blockade resulted in the reduction of donor CD4+ or CD8+ T cells in the periphery by day 30 after transplantation. Paradoxically, the inhibition of GVHD by anti–IL-7Rα antibody treatment resulted in improved long-term thymic and immune function. Blockade of IL-7R by anti–IL-7Rα antibody resulted in elimination of alloreactive T cells, prevention of GVHD, and improvement of donor T-cell reconstitution.
Introduction
Graft-versus-host disease (GVHD) continues to be a limiting factor in the use of clinical hematopoietic stem-cell transplantation (HSCT). GVHD occurs when donor T cells recognize host antigenic disparities expressed on antigen-presenting cells (APCs), resulting in activation of alloreactive T cells and destruction of host tissues. Patients with GVHD develop a wide range of symptoms, including skin rash, diarrhea, liver disease, erythema, and weight loss, which eventually result in death.1–7 Immunosuppressive drug treatment or mature T-cell–depleted bone marrow transplantation (TCD BMT) have been used as effective strategies to prevent GVHD.8,9 However, these strategies can also lead to engraftment failure, a prolonged state of immunodeficiency, and various types of opportunistic infections. Therefore, developing a therapeutic strategy to suppress GVHD without compromising the immune system will be ideal for allogeneic BMT recipients.
IL-7 and Kit ligand (KL; stem-cell factor [SCF]) are the major lymphopoietic cytokines produced in the thymus and BM compartment.10–13 IL-7 induces proliferation, differentiation, and survival of immature T lymphocytes. During normal T-cell development in the thymus, IL-7 produced by thymic epithelial cells (TECs) binds to the cognate IL-7 receptor (IL-7R). The IL-7R is composed of IL-7Rα and common γ subunits and expressed on the surface of immature T-lymphoid progenitor cells. Mutations of the IL-7, IL-7Rα, and γc genes result in defective thymopoiesis and impaired ability to produce T lymphocytes.14–18 Previously we and others have shown that administration of recombinant human IL-7 following histocompatible BMT in murine recipients corrects thymopoietic defects and enhances immune reconstitution, further suggesting the importance of IL-7 in the development of T lymphocytes.19 Besides its thymopoietic effects, IL-7 also promotes expansion and survival of mature naive and memory CD4+ and CD8+ T cells. Recent studies have shown that IL-7–IL-7R interactions in concert with low-affinity interactions between T-cell receptors (TCRs) and self-peptide ligands bound to major histocompatibility complex (MHC) allow proliferation of mature T cells in the periphery.20–26 In addition, IL-7 enhances the survival of alloreactive donor T cells in allogeneic BMT recipients and plays a crucial role in the development and exacerbation of GVHD.27–31
Based on the effects of IL-7 on mature T cells, we investigated whether GVHD could be prevented by a blockade of IL-7Rα with an anti–IL-7Rα monoclonal antibody. Similar to earlier experimental results that we obtained from the genetic model of IL-7 deficiency, we demonstrated that anti-IL-7Rα antibody treatment can successfully prevent GVHD by eliminating donor mature T cells.27 Paradoxically, anti–IL-7Rα antibody treatment did not impair donor-derived thymopoiesis even though IL-7 is critical for the development of T cells. These results indicate that anti-IL-7Rα antibody treatment may be beneficial for prevention of GVHD.
Materials and methods
Mice
Female C57BL/6J (H-2kb, CD45.2), male B6.SJL (H-2kb, CD45.1), male BALB/c (H-2kd Thy 1.2), and male BALB/c (H-2kd Thy 1.1) mice (aged 8 to 10 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were kept in laminar flow cages with autoclaved food and acidified water. The protocol for maintaining animals before and after BMT was approved by the Childrens Hospital Los Angeles Research Institute Animal Care Committee (IACUC).
Bone marrow transplantation procedure
Female recipient H2Kb C57BL/6J mice were given 2 separate doses of radiation (700 cGy on day −1 and 600 cGy on day 0) as previously described.27 The BM from BALB/c (H2Kd Thy 1.1), BALB/c (H2Kd Thy 1.2), or B6.SJL donor mice were obtained by perfusion of the femur, and the lymph nodes (LNs) from BALB/c (H2Kd Thy 1.2) were prepared by mincing of mesenteric, axillary, and inguinal LNs. The donor BM cells were depleted for mature T lymphocytes by immmunomagnetic depletion using rat antimouse Thy 1, CD4, and CD8 monoclonal antibodies (Pharmingen, San Diego, CA) and sheep antirat antibodies conjugated to beads (Dynal, Great Neck, NY). Following irradiation of recipient mice, 1 × 106 TCD BM and 4 × 106 LN cells were transplanted into recipients via tail vein injection.
Administration of anti–IL-7Rα antibody
Antimurine IL-7Rα antibody produced from the ST185 hybridoma clone (gift of Paul Kincade, University of Oklahoma) was purified using a HiTrap Protein G HP antibody isolation kit (GE Healthcare Bio-Sciences, Piscataway, NJ). To test whether anti–IL-7Rα antibody treatment can prevent GVHD, allogeneic BMT-plus-LN recipients were subcutaneously injected weekly with either saline, nonspecific rat IgG (AbD Serotec, Raleigh, NC), or anti-IL-7Rα antibody (100 μg per mouse) from day 0 for 4 weeks. The clinical status of the BMT recipients was evaluated using a GVHD scoring system as previously described (B.C., E.D., A. Toyama, L.B., K.W., manuscript submitted, July 2007).
Cell proliferation assay
To test the anti–IL-7Rα antibody's ability to block IL-7R signaling, 3H-thymidine incorporation assay was performed using the murine IL-7–dependent B-cell line, 2E8 (American Type Culture Collection [ATCC], Manassas, VA). The 2E8 cell line was incubated with either freshly purified anti–IL-7Rα antibody (100 ng/mL) or nonspecific IgG for 30 minutes and treated with rhIL-7 in various different concentrations for 24 hours to measure cell proliferation.
Assessment of GVHD
GVHD severity was assessed using the previously described clinical scoring system (B.C., E.D., A. Toyama, L.B., K.W., manuscript submitted, July 2007). Each BMT recipient was scored weekly for 5 parameters (weight loss, skin integrity, fur texture, mobility, and posture) using a scale of 0 to 2, with 0 for absent or normal, 1 for mildly abnormal, and 2 for severely abnormal. The GVHD clinical index was the sum of the scores for individual criteria.
Histologic analyses
Small intestine, skin, and thymic tissues were fixed in 10% formalin, embedded in parafin, and cut into 5 μm-thick sections for hematoxylin and eosin (H&E) staining. Tissue sections were examined for evidence of GVHD. Immunohistochemical staining of thymic sections were done as previously described.32 The thymus from normal and BMT-recipient mice at day 30 after BMT was embedded in Tissue-Tek OCT compound (SaKura, Torrance, CA) to make frozen sections. Cryosections (5 mm) were immediately fixed with ice-cold acetone and incubated with 10% normal donkey serum (Sigma, St Louis, MO) for 10 minutes to block nonspecific binding of antibodies. Thymic sections were stained with rabbit antimouse keratin 5 (Covance Research Products, Berkeley, CA) and rat antikeratin 8 (Troma-1, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) in 1% donkey serum overnight. As a negative control, normal rabbit or rat serum (Sigma) was used. FITC-conjugated donkey antirat IgG and Cy5-conjugated donkey antirabbit IgG (Jackson Laboratory) were used as secondary antibodies. Thymic tissue sections were viewed with a Leica DM RXA-RF-8 fluorescence microscope (Leica, Bannockburg, IL) using 5×/0.12 NA, HC Plan 10×/0.4NA, or HC Plan 20×/0.7 NA objective lenses for × 50, × 100, and × 200 magnification, respectively. To capture images, a SkyVision-2/VDS-1300 12-bit digital camera (Applied Spectral Imaging, Migdal HaEmek, Israel) with Easy FISH software (Applied Spectral Imaging) was used.
Immunophenotyping
BMT recipients were killed by CO2 narcosis, and the thymus, spleen, and LN cells were removed. Single-cell suspensions of each organ were prepared, and the total cell numbers were determined. A total of 1 × 105 cells were stained with conjugated antibodies directed against Thy1.1, Thy1.2, CD4, CD8, CD44, CD45.1, CD62L, H2Kd, or isotype control monoclonal antibodies (Pharmingen). After staining, cells were washed and analyzed on the FACSCalibur (Becton Dickinson, San Diego, CA). In some experiments, donor LN cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) prior to transplantation to measure the proliferation in vivo of the cells after transplantation. Donor LN cell proliferation was assessed by measuring separate peaks of decreased intensity of CFSE fluorescence upon successive cell division by fluorescence-activated cell sorter (FACS) analysis of the labeled donor CD4 or CD8 T-cell population. When cell numbers were limiting, LN cells from multiple recipients in each experimental group were pooled for analyses.
SRBC immunizations
Normal and recipient mice were immunized with sheep red blood cells (SRBCs) (Colorado Serum, Denver, CO) via intraperitoneal injection at day 44 after BMT, as previously described.19 After 2 weeks, peripheral blood was drawn for primary antibody responses against SRBCs, and the mice were boosted with SRBCs for secondary responses, which were measured 1 week later. The agglutination antibody titers in the serum were determined by serial dilution and incubation with SRBCs in 96-well V-bottom microplates (Corning, Corning, NY).
Statistical analyses
Analyses of survival rates were performed by Winstat Survival Analyses (A-Prompt, Whitehall, PA). Different immunophenotypic populations of cells following transplantation were analyzed by 2-tailed t test with unequal distributions. P values that were less than or equal to .05 were considered statistically significant.
Results
Decreased IL-7–dependent proliferation of 2E8 cell line by anti–IL-7Rα antibody
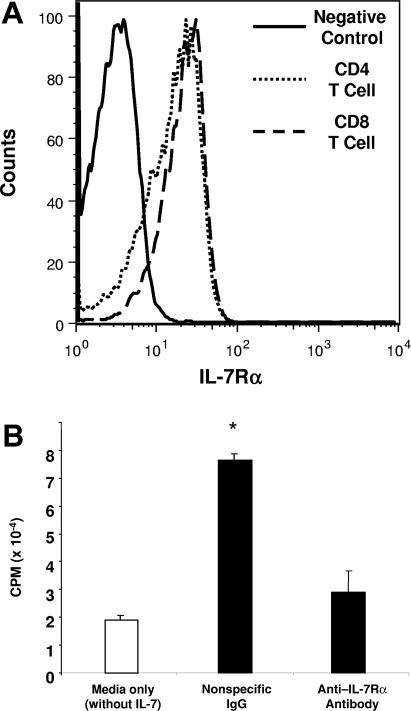
Because our previous data demonstrated that proliferation and maintenance of donor-derived allogeneic mature T cells are reduced in the absence of IL-7, we investigated whether a blockade of IL-7R signaling by anti–IL-7Rα antibody treatment had similar effects.27 To determine whether the anti–IL-7Rα antibody purified from the ST185 hybridoma supernatant binds to the murine IL-7Rα, LN T cells from a BALB/c mouse were incubated in vitro with the anti-IL-7Rα antibody. The purified anti–IL-7Rα antibody bound to both CD4 and CD8 T cells expressing IL-7Rα (Figure 1A). Next we performed a cell proliferation assay using the IL-7–dependent B-cell line, 2E8, to determine if binding of anti–IL-7Rα antibody blocked IL-7–dependent proliferation. After incubating the cell line in media that contain anti–IL-7Rα antibody (100 ng/mL), IL-7 was added and proliferative capacity was measured. Figure 1B shows that anti–IL-7Rα antibody can effectively prevent proliferation of the 2E8 cell line. These results demonstrate that anti–IL-7Rα antibody blocks IL-7R signaling and prevents IL-7–dependent cell proliferation in vitro.
Figure 1.
Blockade of IL-7R signaling by anti–IL-7Rα antibody can effectively prevent proliferation of an IL-7–dependent cell line. Antimurine IL-7Rα antibody purified from the ST185 hybridoma clone was tested for its ability to bind to IL-7Rα and block IL-7 signaling. (A) Lymph node CD4 and CD8 T cells from a BALB/c mouse were incubated in vitro with the anti–IL-7Rα antibody purified from the ST185 hybridoma supernatant. (B) To measure the effect of anti–IL-7Rα antibody on cell proliferation, the IL-7–dependent murine B-cell line, 2E8, was incubated in serum-free media with either nonspecific IgG or the anti-IL-7Rα antibody (100 ng/mL) for 30 minutes before IL-7 stimulation (12.5 pg/mL). For a negative control, the 2E8 cell line was incubated in media without IL-7 and IL-7Rα antibody. Cell proliferation was measured by determining the rate of 3H-thymidine incorporation into DNA for 24 hours after stimulation with or without IL-7. Asterisk indicates significant differences (P < .05) between PBS and anti–IL-7Rα antibody-treated groups.
Anti–IL-7Rα antibody treatment prevents GVHD-related morbidity and mortality
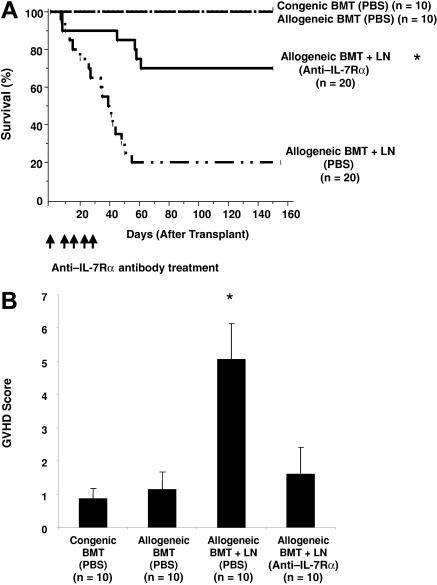
To measure the efficacy of anti–IL-7Rα antibody treatment in the prevention of GVHD, lethally irradiated (1300 cGy) B6 recipient (H2Kb) mice underwent cotransplantation with 1 × 106 TCD BM cells and 4 × 106 LN T cells from allogeneic BALB/c mice (H2Kd). Following transplantation, either 100 μg of IL-7Rα antibody or saline was intraperitionally injected into allogeneic BMT-plus-LN recipients once a week for 4 weeks. In congenic and allogeneic BMT recipients (without LN cell administration), no mortality was observed (Figure 2A). The survival rate of allogeneic BMT-plus-LN recipients treated with saline was approximately 20% by day 150 after transplantation. In contrast, anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients remained significantly higher (70%). Although some of the anti–IL-7Rα antibody-treated animals died of GVHD in the first 61 days after transplantation, no further deaths were observed after day 61.
Figure 2.
Anti–IL-7Rα antibody treatment can successfully prevent GVHD-related mortality and morbidity following allogeneic BM transplantation. Following 1300 cGy total body irradiation (TBI), B6 recipient mice received transplants of 1 × 106 TCD BM and 4 × 106 LN cells from allogeneic BALB/c donor mice and were injected with either PBS or anti–IL-7Rα antibody (100 μg per mouse once a week) subcutaneously for 4 weeks. As controls, 1 × 106 TCD BM cells from either congenic B6.SJL (CD45.1) or allogenic BALB/c (H2Kd) donors were transplanted into lethally irradiated B6 recipients. (A) Survival of anti-IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients is significantly greater than that of the PBS-treated allogeneic BMT-plus-LN recipients (P < .005). Survival over the 150 days after BMT is shown. (B) The severity of GVHD was evaluated by using a GVHD clinical grading system with scoring for 5 clinical criteria: percentage of weight loss, skin integrity, posture, mobility, and fur texture.29 Clinical signs were graded on a scale of 0 to 2, where 0 was absent, 1 was moderate, and 2 was severe, and the individual signs summed. Shown are GVHD clinical index scores at day 30 following BMT. The difference between anti–IL-7Rα antibody- and PBS-treated allogeneic BMT-plus-LN recipients is P < .001*(asterisk).
To further measure the effects of anti–IL-7Rα antibody in preventing GVHD, a GVHD scoring system was used for the first 30 days after BMT to evaluate the clinical status of mice that underwent transplantation (B.C., E.D., A. Toyama, L.B., K.W., manuscript submitted, July 2007). The recipients of either congenic or allogeneic BMT showed no signs of GVHD-related morbidity (Figure 2B). In contrast, allogeneic BMT-plus-LN recipients treated with saline displayed significant weight loss and other evidence of GVHD. Similar to the BMT-only recipients, the anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients had a significantly lower GVHD clinical score compared with saline-treated allogeneic BMT-plus-LN recipients. These results suggest that IL-7Rα antibody treatment reduces GVHD-related morbidity and mortality.
Prevention of GVHD-related tissue inflammation by anti–IL-7Rα antibody
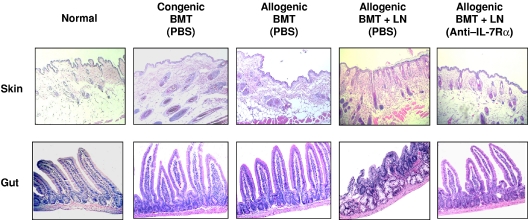
To assess the effects of anti–IL-7Rα antibody on GVHD directly, GVHD target organs were analyzed on day 30 after transplantation. Sections from the skin and small intestine were stained with H&E and assessed for histologic damage. The tissue samples from allogeneic BMT-plus-LN recipients treated with saline displayed histologic evidence of GVHD. The skin samples showed lymphocytic infiltration, hyperkeratosis, and hair loss, and the intestines had villus blunting, lymphocytic infiltration, lamina propria inflammation, crypt destruction, and mucosal atrophy (Figure 3). In contrast, tissue samples from anti-IL-7Rα-treated allogeneic BMT-plus-LN recipients showed no histologic evidence of GVHD and looked similar to those from normal, allogeneic, or congenic BMT recipients. These data provide evidence that anti–IL-7Rα treatment can prevent GVHD-related tissue damage and inflammation.
Figure 3.
Histologic analysis of anti–IL-7Rα antibody-treated allogeneic recipients. Skin and small intestine tissues from the recipients were analyzed at day 30 following BMT. Representative tissue samples from each group of mice were stained with H&E. The skin and small intestine from the PBS-treated allogeneic BMT-plus-LN recipients showed evidence of GVHD with lymphocytic infiltration and inflammation. The tissue sections from anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients had normal histology. See “Materials and methods, Histologic analyses” for image acquisition information.
Decreased numbers of donor T cells in the periphery of anti–IL-7Rα antibody recipients
Because anti–IL-7Rα antibody treatment prevented GVHD, we investigated the effects of anti–IL-7Rα antibody on the survival of donor T cells in vivo. Compared with saline-treated allogeneic BMT-plus-LN recipients, the total number of donor-derived CD4 and CD8 T cells was significantly lower in the spleen and LN of anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients at days 10 and 30 after transplantation (Figure 4). To ensure that antibody treatment did not cause nonspecific IgG effects (eg, Fc receptor blockade), some allogeneic BMT-plus-LN recipients were also treated with nonspecific rat IgG antibody as a control. GVHD lethality was similar between PBS and control IgG-treated recipients (data not shown). The number of donor-derived T cells from the IgG-treated group was not decreased when compared with saline-treated recipients, and the control IgG group had significantly higher numbers of donor CD4 and CD8 T lymphocytes in the LN than anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, the kinetics of the reduction of allogeneic donor T cells in the peripheral blood of anti–IL-7Rα antibody-treated recipients at days 10, 20, and 30 in these experiments showed that the reduction of donor T-cell numbers is progressive over the first 20 to 30 days after transplantation (Figure S2).
Figure 4.
Administration of anti–IL-7Rα antibody results in decreased numbers of donor T cells in the periphery following allogeneic BMT. Analyses of donor-derived peripheral CD4 and CD8 T-cell numbers in anti–IL-7Rα antibody and PBS-treated B6 recipients were performed at day 10 and 30 following allogeneic BMT and LN-cell transplantation. Shown are numbers of donor T lymphocytes at day 10 and day 30 in spleen (A) and LN (B). Asterisk indicates significant differences between PBS- and anti–IL-7Rα-treated groups (spleen and LN at day 10, P < .02; spleen at day 30, P < .02).
To clarify the source of the T cells observed early after transplantation, allogeneic TCD H2Kd Thy1.1+ BM cells underwent cotransplantation with H2Kd Thy1.2+ LN cells to distinguish BM-derived thymic emigrants from adoptively transferred T lymphocytes. BM-derived allogeneic T lymphocytes (Thy 1.1) at day 10 were almost undetectable in the LN of allogeneic BMT-plus-LN recipients. In contrast, most of the donor-derived T lymphocytes detected in the LN were adoptively transferred LN-derived T lymphocytes expressing Thy 1.2 (Figure S3). Therefore, early after transplantation, almost all of the analyzable T cells are derived from the cotransplanted LN cells.
Although anti–IL-7Rα antibody prevents cell proliferation of the 2E8 IL-7-dependent cell line in vitro (Figure 1), the mechanism responsible for the decreased numbers of allogeneic donor T cells in vivo remained unclear. Therefore, we investigated whether inhibition of donor T-cell expansion by anti–IL-7Rα antibody treatment contributes to lower numbers of CD4 and CD8 T cells in the periphery. CFSE-labeled LN cells from BALB/c donor mice were transplanted into lethally irradiated B6 recipients treated with either anti–IL-7Rα antibody or saline. At day 10, donor-derived T cells in the LNs of the recipient mice were analyzed by gating on H2kd-positive donor T cells (Figure 5). Our data demonstrate that anti–IL-7Rα antibody treatment did not completely block proliferation of donor T cells. However, we observed that the frequency of proliferating donor T cells at day 10 in the presence of anti–IL-7Rα antibody was lower than that of saline-treated recipients. The reduction of donor spleen and LN T-cell numbers in anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients is consistent with the lower incidence of GVHD-related mortality we observed (Figure 2A).
Figure 5.
Administration of anti–IL-7Rα antibody results in decreased proliferation of donor-derived T cells. A total of 1 × 106 TCD BM and 4 × 106 CFSE-labeled LN cells from allogeneic BALB/c donors were transplanted into lethally irradiated B6 recipients. At day 10, the frequency of proliferating CFSE-labeled allogeneic donor-derived (H2kd-positive) CD4 and CD8 T cells in LN (to the left of the indicated bar) of the anti–IL-7Rα antibody-treated allogeneic BMT and LN recipients was less than that of the PBS-treated recipients.
Anti–IL-7Rα antibody treatment results in improves GVHD-related thymic atrophy
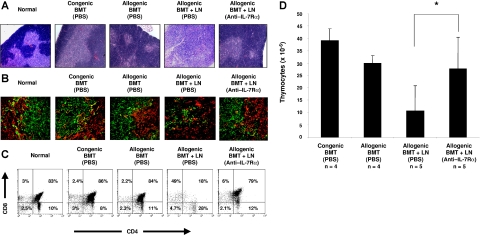
TECs are targets of GVHD in both clinical and experimental allogeneic BMT.31–33 Alloreactive donor T cells are known to damage the host thymic microenvironment. Because anti–IL-7Rα antibody treatment resulted in reduction of donor-derived T cells in the periphery, we investigated whether the thymic architecture from the anti–IL-7Rα antibody-treated recipients was also protected. The relative size of the thymus from saline-treated allogeneic BMT-plus-LN recipients was much smaller than that of the normal thymus and displayed no demarcation between cortical and medullary regions (Figure 6A and data not shown). In contrast, the thymic histology from the anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients did not appear to be different from that of normal, congenic, or allogeneic BMT recipients. Based on the histologic analyses, we determined whether the TECs of the anti–IL-7Rα antibody-treated recipients were normal. Thymic cortical and medullary epithelial cells have been well characterized by differential expression of cytokeratins (K5− K8+ for cortical and K5+ K8− for medullary).35 Corresponding to the H&E data, the thymus from saline-treated allogeneic BMT-plus-LN recipients displayed abnormal cortical and medullary keratin expression patterns (Figure 6B). Both K5− K8+ cortical and K5+ K8− medullary epithelial cells were scattered throughout the thymus, and the absence of distinctive corticomedullary junctions was observed. In contrast, the thymus from anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients displayed normal cytokeratin expression patterns throughout the thymus resembling normal and BMT-only recipients.
Figure 6.
Anti–IL-7Rα antibody treatment does not impair donor-derived thymopoiesis following allogeneic BMT. Both histologic features and donor-derived cell analysis of the thymus from recipient mice were analyzed at day 30 after BMT. Shown are representative thymuses from normal, PBS-treated congenic BM, PBS-treated allogeneic BM, PBS-treated allogeneic BMT-plus-LN, and anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN-cell recipient mice. (A) Similar to normal and BMT-only recipients, the thymus from anti–IL-7Rα–treated allogeneic BMT and LN-cell recipients shows sharp demarcation between cortical (dense blue areas) and medullary (less dense blue areas) regions in H&E staining. Conversely, atrophic thymus from PBS-treated allogeneic BMT-plus-LN recipients displayed disorganized boundaries between cortex and medulla. (B) Thymic tissue sections from recipients receiving transplants were stained with FITC-conjugated anti-K8 (green) and PE-conjugated anti-K5 (red) antibodies to characterize the cortical (K5− K8+) and medullary (K5+ K8−) TECs. (C) Representative FACS analyses of CD4 and CD8 expression by donor-derived thymocytes at day 30 after BMT. (D) The number of donor-derived thymocytes in BMT recipients at day 30. The difference between PBS- and anti–IL-7Rα–treated allogeneic recipients is P < .05 (asterisk). See “Materials and methods, Histologic analyses” for image acquisition information.
The thymic tissue damage in saline-treated allogeneic BMT-plus-LN recipients suggests that donor-derived T-cell development might be also impaired. Therefore, the recipient thymus was analyzed for the relative distributions of donor thymic subpopulations at day 30 after BMT. Shown in Figure 6C are representative flow cytometric analyses of the thymus from BMT recipients. The frequency of CD4+ CD8+ double-positive (DP) cells was greatly diminished, whereas the CD4+ CD8− and CD4− CD8+ single-positive (SP) cells were increased in saline-treated allogeneic BMT-plus-LN recipients when compared with those from congenic or allogeneic BMT recipients. In contrast, the relative frequencies of the donor-derived thymocyte subpopulations in anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients were similar to those seen in normal mice or BMT-only recipients. Correspondingly, the number of donor-derived thymocytes from anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients was significantly higher than saline-treated allogeneic BMT-plus-LN recipients (Figure 6D).
To investigate whether the improvement of donor-derived thymopoiesis following anti–IL-7Rα antibody treatment is solely caused by the suppression of alloreactivity rather than stimulatory or beneficial effects of anti–IL-7Rα antibody, we performed congenic TCD BMT (B6.SJL [H2Kb CD45.1+] donors) in lethally irradiated B6 recipients (H2Kb CD45.2+) and subcutaneously injected them weekly with either anti–IL-7Rα antibody or nonspecific rat IgG (100 μg per mouse) for 4 weeks. At day 30 after BMT, we observed that there is no beneficial effect in congenic hosts treated with anti–IL-7Rα antibody when compared with nonspecific rat IgG-treated congenic recipients. (Figure S4). These results demonstrate that anti–IL-7Rα antibody treatment not only prevents GVHD but also improves donor-derived thymopoiesis by suppression of alloreactivity.
Anti–IL-7Rα antibody treatment improved development of T-cell–dependent antibody responsiveness
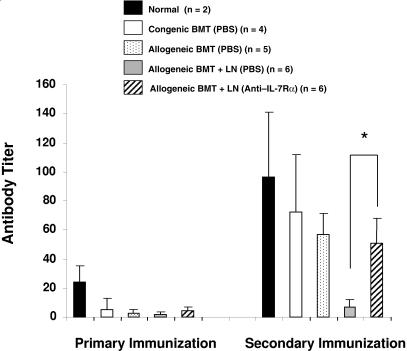
Because anti–IL-7Rα antibody treatment preserved donor-derived thymopoiesis at day 30, we then examined functional T-cell–dependent immunity by immunizing with SRBCs the recipients that received transplants. The mice were immunized with SRBCs on day 44, and the secondary boost was given 2 weeks after primary immunization (day 58). Following immunization, we measured primary and secondary antibody responses directed against SRBCs. The saline-treated allogeneic BMT and LN transplantation group had low anti-SRBC titers after primary and secondary immunization (Figure 7). In contrast, the anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients displayed significantly higher secondary titers than the saline-treated allogeneic BMT-plus-LN recipients, and titers in the anti–IL-7Rα antibody-treated recipients were comparable to those observed in the normal and BMT-only recipients.
Figure 7.
Anti–IL-7Rα antibody treatment results in increased anti-SRBC titer following immunization. Anti-SRBC agglutinating titers were measured 1 week after the primary immunization (at day 44) and the secondary immunization (at day 58) after BMT. Asterisk indicates significant differences between PBS- and anti–IL-7Rα–treated allogeneic BMT-plus-LN recipient groups (P < .006).
Anti–IL-7Rα antibody treatment preserves donor-derived T-lymphoycte populations long-term after allogeneic BMT
Because improved thymic histology and antibody response to SRBC immunization suggest that immune reconstitution is improved in anti–IL7Rα antibody-treated recipients, we further investigated whether anti–IL-7Rα antibody treatment preserves donor T lymphocytes beyond day 30 after allogeneic BMT-plus-LN transplantation. At day 250, we observed that the thymic histology and relative distributions of donor thymic subpopulations from the anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients appeared normal (Figure S5). In addition, the presence of naive donor-derived (CD62L+ CD44−) T lymphocytes in the spleen at later dates indicates the persistence of thymopoiesis. Because all the thymocytes and leukocytes in the spleen (including T cells) are donor derived, our data strongly suggest that anti–IL-7Rα antibody treatment does not affect engraftment or the ability of donor hematopoietic progenitor cells to give rise to T cells.
Discussion
Because IL-7 plays a critical role in mature T-lymphocyte expansion and survival, we investigated whether a blockade of IL-7R signaling by anti–IL-7Rα antibody can prevent the development of GVHD in the allogeneic setting. We observed a significantly lower incidence of GVHD-related mortality in allogeneic BMT-plus-LN recipients treated with anti–IL-7Rα antibody (30%). The surviving mice remained free of GVHD for up to 8 months, even after anti–IL-7Rα antibody treatment was discontinued. In contrast, 80% of saline-treated allogeneic BMT-plus-LN recipients developed GVHD and died by day 50 after transplantation. The overall GVHD clinical index score of recipients treated with anti–IL-7Rα antibody was also significantly lower than that of the saline-treated allogeneic BMT-plus-LN recipients. The anti–IL-7Rα antibody treatment resulted in decreased numbers of donor-derived CD4+ and CD8+ T lymphocytes in the peripheral organs of recipients early after transplantation (before day 30). Taken together, the result of anti–IL-7Rα antibody treatment is similar to that in previous B6.IL-7−/− allogeneic recipients in which the absence of host IL-7 production resulted in prevention of GVHD (B.C., E.D., A. Toyama, L.B., K.W., manuscript submitted, July 2007).
Although IL-7 was initially thought to regulate T and B lymphopoiesis, IL-7 signaling is also important for the peripheral expansion and survival of mature T cells.20–26 Adoptive transfer of naive congenic T lymphocytes into sublethally irradiated IL-7−/− recipients results in minimal expansion of donor T cells, which could be restored by IL-7 administration.24 In addition, IL-7 mediates homeostasis of CD4 and CD8 memory T cells in vivo.20–22 IL-7 signaling also promotes survival of activated allogeneic donor T cells that are prone to undergo activation-induced cell death (AICD) in early stages of GVHD.27 A recent report by Zhang et al demonstrated that persistence of GVHD is caused by donor memory T cells whose homeostatic survival is enhanced by IL-7.30 Because increased circulating levels of endogenous IL-7 are correlated with lymphopenia, a blockade of IL-7R signaling on alloreactive T lymphocytes in the early phases of allogeneic BMT may prevent GVHD by promoting apoptosis and suppressing expansion of host-alloreactive donor T cells.26,36–38 We think that the reduced numbers of donor-derived T cells in antibody-treated animals are mainly due to IL-7Rα blockade function rather than via antibody-mediated cell lysis. The anti–IL-7Rα antibody can effectively inhibit proliferation of the IL-7–dependent pre-B cell line, 2E8, in the presence of IL-7, as previously reported by Kincade et al.39 Here, the kinetics of disappearance of peripheral donor T cells in the anti–IL-7Rα antibody–treated allogeneic BMT-plus-LN recipients was relatively slow compared with what would be expected if the antibody were directly mediating cell lysis. Interestingly, the observed kinetics were similar to the rate of disappearance of donor T cells in our previous allogeneic IL-7−/− recipient models (B.C., E.D., A. Toyama, L.B., K.W., manscript submitted, July 2007). Therefore, our data suggest that disappearance of donor T cells is more likely caused by blockade of IL-7R signaling than by antibody-mediated lysis.
Mice and humans with mutations in the IL-7Rα or IL-7 genes have defects in thymopoiesis and a severe combined immunodeficiency (SCID) phenotype due to lack of production of mature T cells.14–18 Blockade of IL-7R signaling by the anti-IL-7Rα antibody might have been expected to inhibit donor-derived T-cell development in the recipient thymus and delay donor immune reconstitution. Instead, we observed that anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients had improved thymic structure and cellularity compared with saline-treated allogeneic BMT-plus-LN-treated recipients. Similar to the congenic and allogeneic BMT recipients, anti–IL-7Rα antibody-treated recipients displayed normal thymic structure including cortical and medullary demarcation. Donor alloreactive T lymphocytes infiltrate and damage the host thymus in experimental GVHD models.31–33 We hypothesize that the magnitude of the thymic protective effects of the anti–IL-7Rα antibody treatment exceeds any potential inhibitory effect of IL-7R blockade in the thymus. In addition, we demonstrated that the improvement of donor-derived thymopoiesis by anti–IL-7Rα antibody treatment is related to suppression of GVHD rather than a thymic stimulatory effect of either control IgG or IL-7R blockade. This was shown in congenic TCD BMT recipients in which no difference in donor-derived thymopoiesis was observed following nonspecific IgG or anti–IL-7Rα antibody treatment. It is also possible that the thymus-blood barrier excluded anti–IL-7Rα antibody from entering the host thymus and blocking T-cell development.40–42 For example, Ghobrial et al demonstrated that the amount of anti-CD4 or anti-CD8 antibody required for depletion of mature T cells in the periphery was 1000-fold less than that required to cross the thymus-blood barrier to deplete thymocytes.43
Following SRBC immunization, we observed that the response to a neoantigen in anti–IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients was significantly greater than in the saline-treated allogeneic BMT-plus-LN recipients. It is likely that the improvement of T-cell–dependent humoral immunity in the anti–IL-7Rα-treated recipients was caused by donor-derived naive T lymphocytes generated from the recipient thymus. Although the low number of donor T lymphocytes in the spleen and LN of anti–IL-7Rα antibody-treated recipients was observed at day 30 after allogeneic BMT-plus-LN transplantation, the subsequently improved secondary antibody responses to SRBCs are likely to be due to the increased number of peripheral donor T lymphocytes seen after cessation of anti–IL-7R treatment.
Due to the diverse TCR repertoire, naive T cells generated from the thymus have the ability to respond to a wide variety of foreign antigens more effectively than memory T cells with a limited antigenic repertoire.44 The protection of the host thymus against donor alloreactive T cells provided by anti–IL-7Rα treatment resulted in greater immunocompetence, as measured by significantly higher antibody titers directed against SRBCs after immunization. Because IL-7Rα antibody-treated allogeneic BMT-plus-LN recipients showed no signs of GVHD for up to 8 months, it is likely that donor T cells developed from the thymus are not self-reactive.
Even though conventional regimens such as TCD BMT or immunosuppressive therapies can effectively prevent GVHD, delayed immune reconstitution and suppressed immune responses can often lead to fatal infections. In addition, complete depletion of donor T cells increases the risk of graft rejection by residual host T lymphocytes. Here we have demonstrated that blocking of IL-7R signaling following allogeneic BMT-plus-LN prevented GVHD while permitting immune reconstitution. The question of whether anti–IL-7Rα antibody specifically targets the donor T cells responsible for causing GVHD while sparing T cells required for development of tolerance is under investigation.45 Our data demonstrate that transient blockade of IL-7R signaling by anti–IL-7Rα antibody may have therapeutic potential for prevention of GVHD.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL 54729, HL 73104, AI 50765, CA04961) and the T.J. Martell Foundation (K.I.W.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Authorship
Contribution: B.C. designed and performed the research, analyzed data, and wrote the paper; E.P.D. and D.M. performed the research; L.B. analyzed the data; N.S. contributed reagents; and K.I.W. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth Weinberg, Division of Stem Cell Transplantation, Department of Pediatrics, Stanford University School of Medicine, 1000 Welch Rd, Suite 301, Palo Alto, CA 94304; e-mail: kw1@stanford.edu.
References
- 1.Ferrara JL, Deeg HJ. Graft versus host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JML. Cytokine dysregulation as a mechanism of graft-versus-host disease. Curr Opin Immunol. 1993;5:794–799. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- 3.Antin JH, Verra JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 4.Sprent J, Schaefer M, Korngold R. Role of T-cell subsets in lethal graft-versus-host disease (GVHD) directed to class I versus class II H-2 differences. II. Protective effects of L3T4+ cells in anti-class II H-2 differences. J Immunol. 1990;144:2946–2954. [PubMed] [Google Scholar]
- 5.Korngold R, Sprent J. Murine models for graft-versus-host disease. In: Thomas E.D., editor. Bone Marrow Transplantation. Oxford, United Kingdom: Blackwell Scientific Publications; 1994. pp. 220–230. [Google Scholar]
- 6.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprent J, Schaefer M, Gao EK, Korngold R. Role of T cell subsets in lethal graft-versus-host disease (GVHD) directed to class I versus class II H-2 differences. I L3T4+ cells can either augment or retard GVHD elicited by Lyt-2+ cells in class I- different hosts. J Exp Med. 1988;167:556–569. doi: 10.1084/jem.167.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki T. Recent advances in the treatment of graft-versus-host disease. Clin Med Res. 2004;2:243–252. doi: 10.3121/cmr.2.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron F, Storb R. Allogeneic hematopoietic cell transplantation as treatment for hematological malignancies: a review. 2004;26:71–94. doi: 10.1007/s00281-004-0165-3. [DOI] [PubMed] [Google Scholar]
- 10.Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 11.Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;169:707–716. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- 12.Henney CS. Interleukin 7: effects on early events in lymphopoiesis. Immunol Today. 1989;10:170–173. doi: 10.1016/0167-5699(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 13.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 14.Pechon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somberg R, Robinson JP, Felsburg P. T lymphocyte development and function in dogs with X-linked severe combined immune deficiency. J Immunol. 1994;153:4006–4015. [PubMed] [Google Scholar]
- 17.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin 2 receptor γ chain mutation results in X-linked severe combined immune deficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 18.Sudo TS, Nishikawa N, Ohno N, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolotin E, Smith S, Smogoreswka EM, Widmer M, Weinberg KI. Enhancement of thymopoiesis after bone marrow transplant by in vivo IL-7. Blood. 1996;88:1887–1894. [PubMed] [Google Scholar]
- 20.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 21.Goldrath AW, Sivakumar PV, Glaccum M, et al. Cytokine requirements for acute and basal homeostatic proliferation of naïve and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 23.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 24.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maraskovsky E, Teepe M, Morrissey PJ, et al. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 27.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gendelman M, Hecht T, Logan B, Vodanovic-Jankovic S, Komorowski R, Drobyski WR. Host conditioning is a primary determinant in modulating the effect of IL-7 on murine graft-versus-host disease. J Immunol. 2004;172:3328–3336. doi: 10.4049/jimmunol.172.5.3328. [DOI] [PubMed] [Google Scholar]
- 29.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002;100:2642–2649. doi: 10.1182/blood-2002-04-1082. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol. 2005;174:3051–3058. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg K, Blazar BR, Wagner J, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 32.Min D, Panoskaltsis-Mortari A, Kuro-O M, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seemayer TA, Lapp WS, Bolande RP. Thymic involution in murine graft versus host reaction: epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977;88:119–133. [PMC free article] [PubMed] [Google Scholar]
- 34.Krenger W, Rossi S, Piali L, Hollander GA. Thymic atrophy in murine acute graft-versus-host disease is effected by impaired cell cycle progression of host pro-T and pre-T cells. Blood. 2000;1996:347–354. [PubMed] [Google Scholar]
- 35.Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci U S A. 1998;95:11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–788. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 37.Llano A, Barretina J, Gutierrez A, et al. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J Virol. 2001;75:10319–10325. doi: 10.1128/JVI.75.21.10319-10325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 39.Borghesi LA, Yamashita Y, Kincade PW. Heparan sulfate proteoglycans mediate interleukin-7-dependent B lymphopoiesis. Blood. 1999;93:140–148. [PubMed] [Google Scholar]
- 40.Frohlich E. Structure and function of blood-tissue barriers. Dtsch Med Wochenschr. 2002;127:2629–2634. doi: 10.1055/s-2002-35932. [DOI] [PubMed] [Google Scholar]
- 41.von Gaudecker B. Functional histology of the human thymus. Anat Embryol. 1991;183:1–15. doi: 10.1007/BF00185830. [DOI] [PubMed] [Google Scholar]
- 42.Henry L, Durrant TE, Anderson G. Pericapillary collagen in the human thymus: implications for the concept of the ‘blood-thymus’ barrier. J Anat. 1992;181:39–46. [PMC free article] [PubMed] [Google Scholar]
- 43.Ghobrial RRM, Boublik M, Winn BH, Auchincloss H. In vivo use of monoclonal antibodies against murine T cell antigens. Clin Immunol Immunopathol. 1989;52:486–506. doi: 10.1016/0090-1229(89)90162-1. [DOI] [PubMed] [Google Scholar]
- 44.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 45.Liu W, Putnam AL, Xu-yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.