Abstract
Thymic-derived natural T-regulatory cells (nTregs) are important for the induction of self-tolerance and the control of autoimmunity. Murine CD4+CD25−Foxp3− cells can be induced to express Foxp3 after T-cell receptor (TCR) activation in the presence of transforming growth factor β (TGFβ) and are phenotypically similar to nTregs. Some studies have suggested that TCR stimulation of human CD4+CD25− cells results in the induction of transient expression of FOXP3, but that the induced cells lack a regulatory phenotype. We demonstrate here that TCR stimulation alone was insufficient to induce FOXP3 expression in the absence of TGFβ, whereas high levels of FOXP3 expression could be induced in the presence of TGFβ. Although FOXP3 expression was stable, the TGFβ-induced FOXP3+ T cells were neither anergic nor suppressive and produced high levels of effector cytokines. These results suggest that even high levels of FOXP3 expression are insufficient to define a human CD4+ T cell as a T-regulatory cell.
Introduction
Thymic-derived natural T-regulatory cells (CD4+CD25+Foxp3+; nTregs) are important for the induction of self-tolerance and the control of autoimmunity.1 Mutations in the FOXP3 transcription factor result in a rare human disorder called immune dysregulation/polyendocrinopathy/enteropathy/X-linked syndrome (IPEX), which is characterized by multiple autoimmune diseases.2,3 Patients with this condition typically die by early infancy or childhood. A similar condition consisting of a fatal lymphoproliferative disorder with multiorgan infiltration occurs in the scurfy mouse as a result of a deletion of the Foxp3 forkhead domain.4,5 Deficiencies in nTreg numbers or function have been postulated to play a role in susceptibility to several autoimmune diseases.6 It is essential to study and understand the functions of FOXP3+ nTregs, but human nTregs have proven difficult to isolate because CD25 cannot be readily used as a marker for FOXP3+ cells. The CD4+CD25+ population contains mostly cells that are FOXP3− and express intermediate to low levels of CD25.7
Expression of Foxp3 is considered the definitive marker for mouse nTregs because Foxp3 is not induced during the activation of non–T-regulatory cells.8 Several studies have suggested that this is not the case in humans because T-cell receptor (TCR) stimulation alone with anti-CD3 and anti-CD28 has been reported to induce FOXP3 expression in human CD4+CD25− cells.9–15 In one study, these cells expressed a T-regulatory phenotype,9 whereas several other studies demonstrated that the induction of FOXP3 did not correlate with anergy or suppressive function.10,14,15 There are several problems with these studies. In many, it was difficult to quantify the purity of the starting population, rendering it difficult to distinguish induction of FOXP3 from selection of a few FOXP3+ nTregs present in the starting population. Several studies determined the expression of FOXP3 after induction at the population level using quantitative PCR, whereas others measured FOXP3 at the single-cell level using an anti-FOXP3 monoclonal antibody (mAb), PCH101, that may give nonspecific staining.
A second potential difference between the regulation of FOXP3 expression in the mouse and human is that mouse CD4+CD25−Foxp3− cells can be induced to express Foxp3 when activated in vitro by TCR stimulation in the presence of transforming growth factor β (TGFβ).16,17 TGFβ-induced mouse Tregs appear to resemble nTregs in all their phenotypic and functional properties. They exert potent suppressor function in vitro and can prevent or control disease in vivo.18,19 Only one study has suggested that TGFβ can induce FOXP3 in human CD4+CD25− cells, but the analysis was at a population level and the function of the induced cells was not assessed.17
In this study, we re-examined the requirements for de novo induction of FOXP3 in nonregulatory human CD4+FOXP3− T cells. Using single-cell analysis with multiple mAbs to FOXP3 as well as quantitative PCR, we demonstrate that human CD4+FOXP3− cells resemble mouse CD4+Foxp3− cells. TCR stimulation alone was insufficient to induce FOXP3 expression in the absence of TGFβ, although high levels of FOXP3 could be induced in the presence of TGFβ. FOXP3 expression in the TGFβ-induced cells was stable for several weeks in culture without TGFβ but did require the presence of interleukin-2 (IL-2). In contrast to TGFβ-induced mouse CD4+Foxp3+ cells that were both anergic and suppressive in vitro, human induced CD4+FOXP3+ cells were neither anergic nor suppressive and produced high levels of effector cytokines. The failure of human TGFβ-induced CD4+FOXP3+ cells to exhibit regulatory activity raises the possibility that even high levels of FOXP3 expression are insufficient to define a cell as a Treg and that other factors, present in mouse CD4+ T cells, may be required to act in concert with FOXP3.
Materials and methods
Cell purification
Peripheral blood was obtained from healthy adult donors by the Department of Transfusion Medicine at the National Institutes of Health. Cord blood was collected from term placentas at Shady Grove Adventist Hospital (Gaithersburg, MD). The acquisition of blood products was approved according to the Institutional Review Boards (IRBs) of these 2 institutions and in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were prepared over Ficoll-Paque Plus gradients (GE Healthcare, Piscataway, NJ). The CD4+ cells were enriched over the autoMACS cell separator by positive selection with human CD4 microbeads (both from Miltenyi Biotec, Auburn, CA). The cells were labeled with CD4 fluorescein isothiocyanate (FITC), CD45RA Tricolor, and CD25 APC purchased from Invitrogen (Carlsbad, CA) and CD127 phycoerythrin (PE) from BD Biosciences PharMingen (San Diego, CA) and sorted with either the FACSVantage DiVa or FACSAria flow cytometer (both from BD Biosciences, San Jose, CA). Likewise, the CD4+CD25hi cells were obtained by labeling the cells with CD4 FITC and CD25 PE and fluorescence-activated cell sorting on the top 2% of CD25hi. The postsort purity for CD4+CD25−CD127+CD45RA+ and CD4+CD25−CD127+CD45RA− cells was higher than 97% and the FOXP3 purity for CD4+CD25hi cells was higher than 90%.
In vitro cell activation
The cells sorted by FACS were activated in vitro with plate-bound anti-CD3 and 2 μg/mL soluble anti-CD28 (BD PharMingen) with or without 5 ng/mL transforming growth factor β1 (TGFβ1; Peprotech, Rocky Hill, NJ) in 24-well culture plates (Corning Life Sciences, Acton, MA) at 500,000 cells per well. The culture medium consisted of X-VIVO 20 (Lonza, Walkersville, MD) with or without 5% heat-inactivated autologous serum and 50 IU/mL IL-2 (Hoffman-LaRoche, Nutley, NJ). The culture plate was coated overnight at 4°C with 10 μg/mL OKT3 (Ortho Biotech Products, Bridgewater, NJ). In some experiments, 50 μg/mL neutralizing anti-TGFβ antibody was used (clone 1D11; R&D Systems, Minneapolis, MN). The cells were stimulated for 5 days then washed and transferred to new wells with fresh culture medium containing 50 IU/mL IL-2. The cells were split and fresh culture medium replaced every 2 days. Otherwise, the CD4+CD25−CD127+CD45RA+ cells were activated with either CD3-depleted PBMC, immature monocyte-derived dendritic cells, or mature monocyte-derived dendritic cells in the presence of 1 μg/mL OKT3 and 50 IU/mL IL-2 with or without 5 ng/mL TGFβ1.
Generation of antigen-presenting cells
The CD3-depleted PBMC was generated by incubating PBMC with human CD3 microbeads (Miltenyi Biotec) and running it over the autoMACS with the depletion-sensitive program. The dendritic cells (DCs) were generated from monocyte-enriched peripheral blood obtained from our blood bank and further purified over the autoMACS with positive selection program using human CD14 microbeads (Miltenyi Biotec). The monocyte purity was higher than 99% based on CD14 expression. The monocytes were cultured in X-VIVO 15 (Lonza Walkersville, Inc.) plus 1% heat-inactivated autologous serum with 800 U/mL granulocyte macrophage–colony-stimulating factor (GM-CSF) and 1000 U/mL IL-4 (Peprotech). Fresh medium with GM-CSF and IL-4 was added to cultures on days 3 and 5. For differentiation into mature DCs, the day 5 cultures were additionally stimulated with 10 ng/mL IL-1β, 10 ng/mL tumor necrosis factor–α (TNF-α), 10 ng/mL IL-6 (Peprotech), and 1 μg/mL prostaglandin E2 (Sigma-Aldrich, St. Louis, MO). On day 7, the immature and mature DCs were used for T-cell stimulation.
FACS analysis
FACSCalibur was used for data acquisition, and the data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). For staining of FOXP3, the cells were fixed and permeabilized using a Fixation/Permeabilization kit according to the manufacturer's protocol (eBioscience, San Diego, CA). The FOXP3 was stained with either PCH101 PE (eBioscience) or 259D Alexa Fluor 647 (Biolegend, San Diego, CA) anti-FOXP3 antibody and isotype control.
For analysis of intracellular cytokine production, CD45RA+ cells activated on day 5 were rested in 50 IU/mL IL-2 culture medium for 2 days and restimulated for 5 hours with 25 ng/mL phorbol 12-myristate 13-acetate (PMA) and 250 ng/mL ionomycin (Sigma-Aldrich), along with 3 μg/mL brefeldin A (eBioscience). Afterward, the cells were fixed and permeabilized with an eBioscience kit and stained for FOXP3 with 259D Alexa Fluor 647 and for cytokines with anti-interferonγ (IFNγ) FITC, IL-2 PE, and IL-10 PE purchased from Invitrogen.
Quantitative real-time polymerase chain reaction analysis
Total RNA was extracted from cells with an RNeasy Plus Kit (Qiagen, Valencia, CA). Reverse transcription-polymerase chain reaction (RT-PCR) was performed with approximately 1 μg of isolated RNA for cDNA synthesis using SuperScript II RNase H-reverse transcriptase (Invitrogen). Real-time PCR was performed in triplicate according to the TaqMan Universal 2× master mix and run on the ABI/PRISM 7900 Sequence Detector System (Applied Biosystems, Foster City, CA). The amount of FOXP3 mRNA expression was normalized to the 18S rRNA and calculated according to the comparative cycle threshold (Ct) method as described by Applied Biosystems. FOXP3 primers and probe (Integrated DNA Technologies, Coralville, IA) were as follows: forward primer, 5′-GCA CCT TCC CAA ATC CCA GT-3′; reverse primer, 5′-GGC CAC TTG CAG ACA CCA T-3′; and fluorogenic probe (5-carboxyfluorescein/5-carboxytetramethylrhodamine), 5′-CAG GAA GGA CAG CAC CCT TTC GGC-3′.
FOXP3 siRNA experiments
FOXP3 siRNA was purchased from Invitrogen (Stealth Select RNAi). FOXP3 oligonucleotide HSS121456 was used as nonsilencing siRNA and oligonucleotide HSS121458 was used as silencing siRNA. To transfect the CD45RA+ cells, 200 pmol of siRNA was mixed with 100 μL of the human T-cell Nucleofector solution and 5 × 106 cells were resuspended in this mixture. The cell suspension was immediately electroporated by the Nucleofector II instrument (Amaxa Biosystems, Gaithersburg, MD). The transfected CD45RA+ cells were rested in 50 IU/mL IL-2 culture medium for 24 hours before being stimulated with anti-CD3/anti-CD28 and 50 IU/mL IL-2 in the presence of 50 μg/mL anti-TGFβ or 5 ng/mL TGFβ1 for 5 days and stained for FOXP3 with PCH101.
FOXP3 stability experiments
The CD45RA+ cells stimulated on day 5 were rested in 50 IU/mL IL-2 culture medium for 2 days then washed and replaced with fresh IL-2 culture medium with or without 50 μg/mL anti-TGFβ. Otherwise, the cells were placed in culture medium without IL-2 and 10 μg/mL anti-IL2 (clone 5334; R&D Systems) along with either 50 ng/mL IL-1β, TNFα, IFNγ, IL-4, IL-6, IL-7, IL-10, IL-15 (Peprotech) or without any cytokine. After 48 hours, the cells were stained for FOXP3 with 259D.
Proliferation assays
CD45RA+ cells were activated with or without TGFβ1 and CD4+CD25hi nTregs were activated without TGFβ1 for 5 days and rested for 2 days in IL-2 culture medium. The cells were washed 3 times in phosphate-buffered saline before using them for the suppression assay. Their suppressive functions were tested on day 7 with 5 × 104 fresh allogeneic CD4+CD25− responder cells, sorted by FACS, stimulated with 5 × 104 irradiated (4000 rads) autologous CD3-depleted PBMC, and 0.5 μg/mL OKT3 alone or with non–TGFβ-treated CD45RA+, TGFβ-treated CD45RA+, activated CD4+CD25hi, or fresh CD4+CD25hi cells. The cells were cultured for 4 days in 96-well flat-bottomed plates (Corning Life Sciences) and pulsed with [3H]thymidine (1 μCi/well) for the last 16 hours. In similar experiments, fresh allogeneic CD4+CD25−, non–TGFβ-treated CD45RA+, TGFβ-treated CD45RA+, or fresh CD4+CD25hi cells were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and stimulated with CD3-depleted PBMC and 0.5 μg/mL OKT3 alone or at 1:1 ratio coculture with CD4+CD25− responder cells. The cells were cultured for 5 days, and CFSE dilution was analyzed by FACS.
Results
Induction of FOXP3 expression in naive human CD4+ T cells was TGFβ-dependent
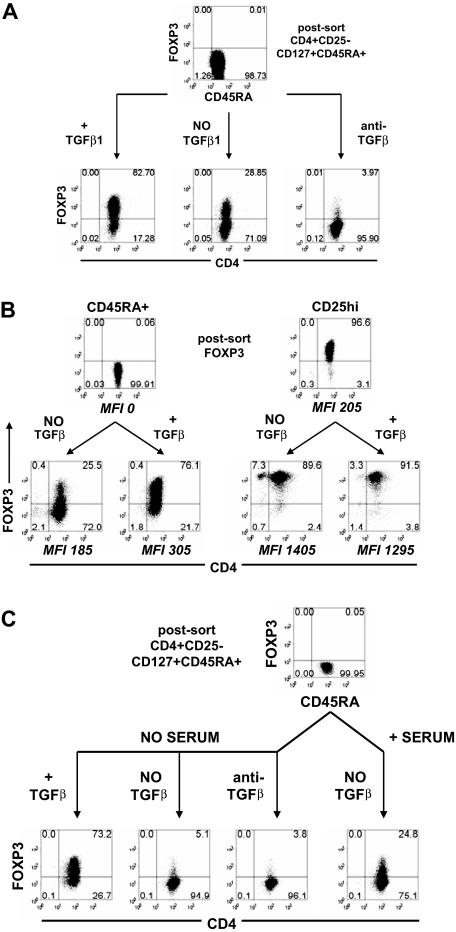
To better define the roles of TCR stimulation and TGFβ in the induction of FOXP3, we purified naive human CD4+CD25−CD127+CD45RA+ T cells by FACS. These cells will be referred to as CD45RA+ cells. We used the CD127 marker to minimize any contaminating CD4+CD25−FOXP3+ nTregs that have been shown to express low to absent level of CD127.20 The resultant population contained less than 0.1% FOXP3+ nTregs as assessed by staining with mAb 259D (Figure 1A). After in vitro stimulation for 5 days with anti-CD3/anti-CD28 and IL-2 in culture medium containing 5% autologous serum, approximately 30% of the cells expressed FOXP3, and this percentage could be increased to approximately 80% in the presence of exogenous TGFβ1. Five-day stimulation gave optimal induction of FOXP3 expression. When the cells were stimulated in the presence of a neutralizing anti-TGFβ mAb, less than 4% of the cells expressed FOXP3. TGFβ1 had no effect on the increase in the level of FOXP3 as defined by mean fluorescence intensity (MFI) in CD4+CD25hi nTregs (Figure 1B). The level of FOXP3 induced in the CD45RA+ cells was never as high as that seen in the stimulated nTregs but was higher than that observed in unstimulated nTregs.
Figure 1.
Induction of FOXP3 in naive human CD4+ T cells was TGFβ-dependent. (A) Flow cytometric analyses of FOXP3 with 259D on postsorted and day 5-activated CD45RA+ cells treated without exogenous TGFβ1 with or without anti-TGFβ mAb or with TGFβ1. (B) FOXP3 expression on postsorted and day 5-activated CD45RA+ cells and CD25hi nTregs treated with and without TGFβ1. The level of FOXP3 expression for each population is represented by the mean fluorescence intensity (MFI) gating on FOXP3+ cells. (C) FOXP3 expression on postsorted and day 5-activated CD45RA+ cells cultured in the presence of serum without exogenous TGFβ or in the absence of serum with exogenous TGFβ1 or without exogenous TGFβ1 with or without anti-TGFβ. Data above are representative of 3 independent experiments. The numbers in each quadrant represent the percentage of cells expressing the antigen.
These results suggest that the induction of FOXP3 in the absence of exogenous TGFβ is mediated by TGFβ present in the serum or produced by the responding T cells. When CD45RA+ cells were stimulated in serum-free medium, approximately 5% of the cells expressed FOXP3 and the addition of anti-TGFβ had little effect (Figure 1C). In contrast, when exogenous TGFβ1 was added to the serum-free medium, more than 70% of the stimulated cells expressed FOXP3. These data are consistent with the results of studies with naive mouse CD4+ T cells that have shown that TCR activation alone does not induce Foxp3 and that the induction of Foxp3 requires TGFβ.
Memory T cells were resistant to TGFβ-induced FOXP3 expression
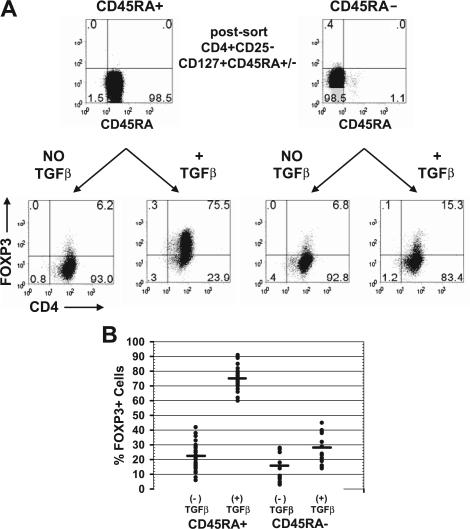
To determine whether all CD4+FOXP3− cells could be induced to express FOXP3, we compared the susceptibility of CD4+CD25−CD127+CD45RA+ and CD4+CD25−CD127+CD45RA− cells to the induction of FOXP3 with TGFβ. It was again important to analyze only CD127+ T cells, in that the majority of the nTregs are CD45RA−CD127− cells.21 The postsort level of FOXP3 was consistently less than 0.1% for the CD45RA+ and less than 1% for the CD45RA− population. Upon activation of these cells with anti-CD3/anti-CD28, IL-2, and TGFβ1 for 5 days, approximately 75% of the CD45RA+ population expressed FOXP3 (Figure 2A). In contrast, only approximately 15% of the CD45RA− population could be induced to express FOXP3 with TGFβ. Similar results were observed from 30 different donors with an average of 22% (range, 6%-42%) of the CD45RA+ cells expressed FOXP3 without exogenous TGFβ and an average of 75% (range, 60%-91%) expressed FOXP3 in the presence of TGFβ1 (Figure 2B). In contrast, only an average of 16% (range, 3%-28%) of the CD45RA− cells expressed FOXP3 without exogenous TGFβ and an average of 28% (range, 14%-45%) expressed FOXP3 with TGFβ. Because naive CD4+ cells were more susceptible to the induction of FOXP3 by TGFβ1 and to minimize any potential contaminating FOXP3+CD25− nTregs, we used the CD4+CD25−CD127+CD45RA+ population for all of our experiments.
Figure 2.
Memory T cells were resistant to TGFβ induction of FOXP3 expression. (A) Flow cytometric analyses of FOXP3 with 259D on postsorted and day 5-activated CD45RA+ or CD45RA− cells treated with and without exogenous TGFβ1. Numbers in each quadrant as in Figure 1. (B) The average percentage and range of FOXP3 induction on day 5-activated CD45RA+ cells from 30 different healthy adult donors and CD45RA− cells from 15 of these donors treated with and without exogenous TGFβ1.
Anti-FOXP3 mAb PCH101 was nonspecific for human FOXP3
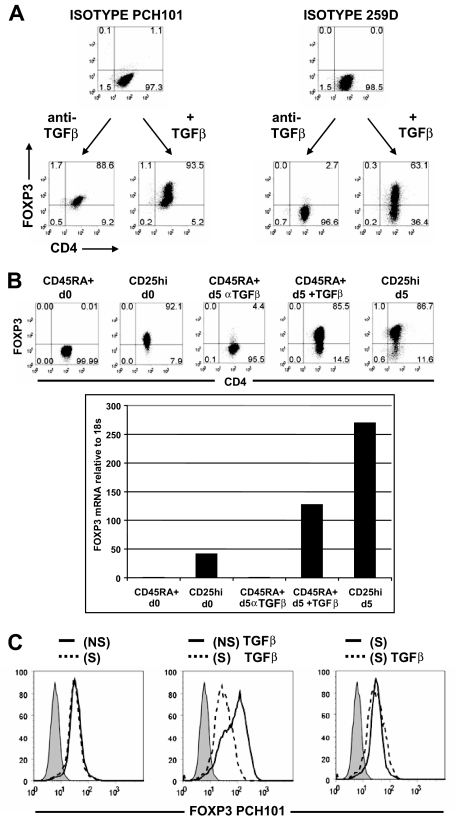
Our results (ie, that only approximately 22% [range, 6%-42%] of CD45RA+ cells could be induced to express FOXP3 without exogenous TGFβ) are contrary to those of several published studies, indicating that the majority expressed FOXP3 when stained with anti-FOXP3 mAb PCH101.13–15 To investigate this discrepancy, activated CD45RA+ cells were stained in parallel with PCH101 and 259D. Based on the isotype control, almost all of the cells activated without TGFβ expressed FOXP3 when stained with PCH101, and 2 peaks of different intensities were seen when the cells were cultured with exogenous TGFβ (Figure 3A). When analyzed with 259D and its isotype control, only a few cells expressed FOXP3 when stimulated without TGFβ, and more than 60% expressed FOXP3 with exogenous TGFβ. Two other anti–human FOXP3 mAbs, 206D and 236A/E7, gave results similar to those seen with 259D (data not shown).
Figure 3.
Anti-FOXP3 mAb, PCH101, was nonspecific for FOXP3 on activated CD4+ T cells. (A) Day 5-activated CD45RA+ cells treated with anti-TGFβ or with exogenous TGFβ1 were costained with PCH101 and 259D anti-FOXP3 mAbs or isotype controls. (B) Flow cytometric with 259D and real-time PCR analyses of FOXP3 protein and mRNA on unstimulated CD45RA+ cells (CD45RA + d0), unstimulated CD25hi nTregs (CD25hi d0), day 5 non–TGFβ-treated CD45RA+ cells (CD45RA + d5 αTGFβ), day 5 TGFβ-treated CD45RA+ cells (CD45RA + d5 + TGFβ), and day 5-stimulated CD25hi nTregs (CD25hi d5). Numbers in each quadrant as in Figure 1. (C) Flow cytometric analyses of FOXP3 with PCH101 and isotype control (shaded histogram) on CD45RA+ cells transfected with nonsilencing (NS, ——) or silencing (S, - - -) FOXP3 siRNA and activated for 5 days without TGFβ1 (left panel) or with TGFβ (center panel). Right panel is an overlay of TGFβ-treated CD45RA+ cells (- - -) and non-TGFβ-treated CD45RA+ cells (——) transfected with silencing FOXP3 siRNA. Data above are representative of 3 independent experiments.
To verify that activated cells stained nonspecifically with PCH101, we also measured FOXP3 expression by quantitative PCR. In the presence of anti-TGFβ, the level of FOXP3 mRNA and protein in CD45RA+ cells stimulated on day 5 was very low and was similar to that detected in the starting population of CD45RA+ cells (Figure 3B). When the CD45RA+ cells were stimulated with exogenous TGFβ1, there was a greater than 100-fold increase in FOXP3 mRNA. However, the level of FOXP3 mRNA and protein in the activated TGFβ-treated CD45RA+ cells was always less than activated nTregs but higher than unstimulated nTregs.
Similar conclusions could be drawn when CD45RA+ cells were transfected with nonsilencing or silencing FOXP3 siRNA and then stimulated in the presence or absence of TGFβ. The reactivity of PCH101 with the cells activated without TGFβ was not reduced by the FOXP3 siRNA (Figure 3C left panel). Furthermore, when the cells were stimulated with exogenous TGFβ, only the bright peak stained with PCH101 was reduced by siRNA treatment, whereas the less bright population was not susceptible to the siRNA and resembled the level of reactivity seen without TGFβ (Figure 3C center and right panels). Taken together, these studies indicate that PCH101 is an unreliable indicator of FOXP3 expression in human activated T cells and gives a false conclusion that FOXP3 represents an activation marker on all activated human CD4 T cells.13–15
TGFβ-induced FOXP3 expression was stable
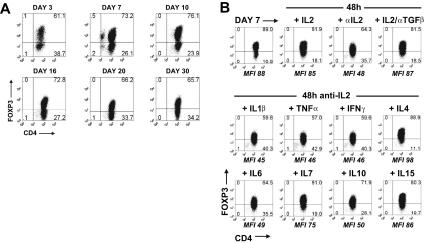
When FOXP3 expression was induced by stimulation of CD45RA+ cells with TGFβ for 5 days and the cells were then washed and maintained in IL-2 culture medium, the percentage of cells staining for FOXP3 was remarkably stable over a 30-day period (Figure 4A). The continued presence of IL-2 was important, in that neutralization of IL-2 over a 48-hour period resulted in a decrease in the percentage and MFI of FOXP3+ cells (Figure 4B). No decrease in cell viability was seen during this period. In contrast, neutralization of TGFβ, in the presence of IL-2, had no effect on the percentage of FOXP3+ cells. It is noteworthy that other cytokines (IL-4, IL-7, IL-15) that use the common γ-chain as part of their receptor complex were also able to maintain FOXP3 expression in the absence of IL-2, whereas IL-1β, TNFα, IFNγ, IL-6, and IL-10 had no effect.
Figure 4.
TGFβ-induced FOXP3 expression was stable and requires IL-2 or other common γ cytokines for maintenance. (A) Flow cytometric analyses of FOXP3 with 259D on TGFβ-treated CD45RA+ cells over a 30-day period in the presence of IL-2. (B) FOXP3 expression on TGFβ-treated CD45RA+ cells at day 7 and 48 hours later after cultured with IL-2 with or without anti-TGFβ or with neutralizing anti-IL2 mAb with or without IL-1β, TNFα, IFNγ, IL-4, IL-6, IL-7, IL-10, or IL-15. The MFI values are derived from the gating of the FOXP3+ population. Data above are representative of 3 independent experiments. Numbers in each quadrant as in Figure 1.
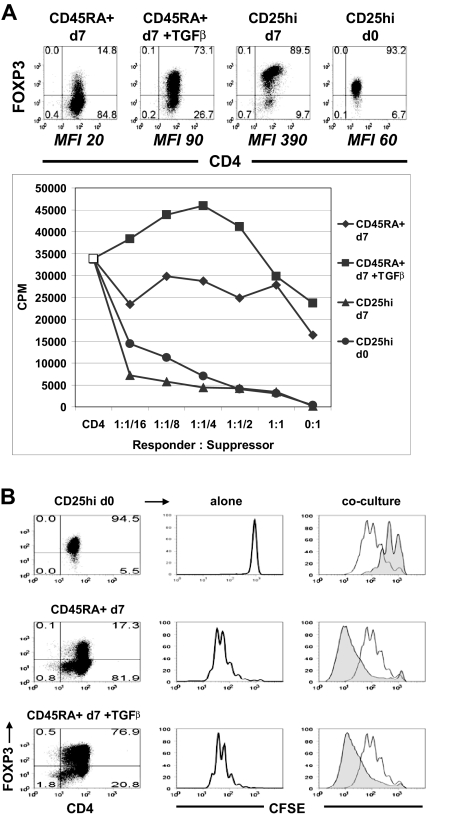
TGFβ-induced FOXP3+ T cells were neither anergic nor suppressive
The phenotypic characteristics of the TGFβ-induced FOXP3+ cells were very similar to thymic-derived nTregs. They stably expressed both isoforms of FOXP3, down-regulated expression of CD127, and up-regulated expression of CTLA-4 (data not shown). To evaluate their regulatory potential, CD45RA+ cells were activated in vitro with TGFβ for 5 days and rested in IL-2 culture medium for 2 days. FACS-purified CD4+CD25hi nTregs also were activated for 5 days and rested for 2 days in IL-2. On day 7, freshly isolated allogeneic CD4+CD25− cells were stimulated with autologous CD3-depleted PBMC and anti-CD3 in the presence of non-TGFβ-treated CD45RA+, TGFβ-treated CD45RA+, activated CD25hi nTregs, or freshly isolated CD25hi nTregs. The percentage and levels of expression of FOXP3 in these populations are shown in the top panel of Figure 5A. Both activated and fresh nTregs failed to proliferate when stimulated with anti-CD3 and were potent inhibitors of the proliferative responses of CD4+CD25− cells as assayed by [3H]thymidine incorporation and CFSE dilution (Figure 5). In marked contrast, the TGFβ-treated FOXP3+ cells were similar to the non–TGFβ-treated FOXP3− cells in that they proliferated when stimulated and failed to suppress responder CD4+CD25− cells. We have allowed these TGFβ-treated FOXP3+ cells to be rested in IL-2 culture medium until day 14, but they still lacked suppressive and anergic functions (data not shown).
Figure 5.
TGFβ-induced FOXP3+ T cells were neither anergic nor suppressive. (A) Suppression assay of allogeneic CD4+CD25− responder cells cultured alone (CD4, □) or with non-TGFβ-treated CD45RA+ cells (CD45RA + d7, ♦), TGFβ-treated CD45RA+ cells (CD45RA + d7 + TGFβ, ■), activated CD25hi nTregs (CD25hi d7, ▴), or fresh CD25hi nTregs (CD25hi d0, ●). The top panel represents the percentage and MFI of the FOXP3+ cells. (B) Suppression assay of CFSE-labeled allogeneic CD4+CD25− cells cultured alone (right column, clear histogram) or at 1:1 ratio (right column, shaded histogram) with fresh CD25hi nTregs (CD25hi d0, right top panel), non–TGFβ-treated CD45RA+ cells (CD45RA + d7, right middle panel) or TGFβ-treated CD45RA+ cells (CD45RA + d7 + TGFβ, right bottom panel). Center column represents proliferation of CFSE-labeled fresh CD25hi (top panel), non–TGFβ-treated CD45RA+ (center panel), or TGFβ-treated CD45RA+ cells (bottom panel). Left column represents the FOXP3 expression of each population. Data above are representative of 3 independent experiments. Numbers in each quadrant as in Figure 1.
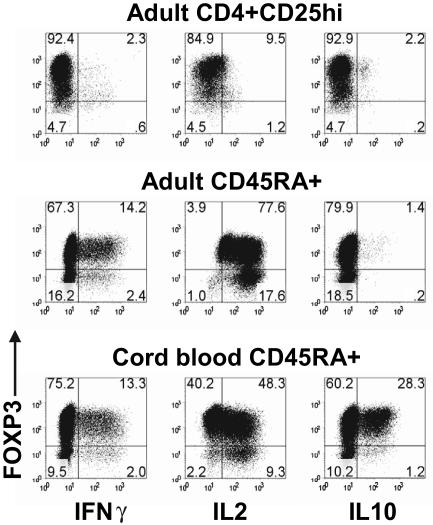
TGFβ-induced FOXP3+ T cells produced IL-2 and IFN-γ
One of the problems in the interpretation of the above studies is that FOXP3 expression was only seen in approximately 75% to 80% of the TGFβ-treated CD45RA+ cells. It remains possible that cytokine production by the FOXP3− cells was inducing proliferation of the FOXP3+ cells and masking their anergic state and their suppressive capacity. We were unable to increase the percentage of FOXP3+ cells by sorting on CD25 or CD127, because both FOXP3+ and FOXP3− CD45RA+ cells from the TGFβ-stimulated cultures expressed similar levels (data not shown). We therefore restimulated the TGFβ-treated CD45RA+ cells with PMA and ionomycin and examined cytokine production at the single cell level by intracellular staining. Although the activated CD4+CD25hi nTregs were anergic, both the FOXP3+ and FOXP3− CD45RA+ cells from the TGFβ-stimulated cultures produced significant amount of IFN-γ and IL-2 (Figure 6).
Figure 6.
TGFβ-induced FOXP3+ T cells produced IL-2 and IFNγ. Flow cytometric analyses of FOXP3, IFNγ, IL-2, and IL-10 on day 7 activated adult CD4 + CD25hi nTregs, adult TGFβ-treated CD45RA+ cells, and cord-blood TGFβ-treated CD45RA+ cells restimulated with PMA and ionomycin. Data are representative of 3 independent experiments. Numbers in each quadrant as in Figure 1.
It is also possible that the CD45RA+ cells present in adult peripheral blood are not truly naive and are in part resistant to some of the inductive effects of TGFβ or to FOXP3-mediated T-regulatory functions. We therefore isolated cord blood CD45RA+ T cells and induced FOXP3 expression using the same protocol described for adult CD45RA+ T cells. Although the majority of the cord blood in CD45RA+ cells could be converted to FOXP3+ cells, they were neither anergic nor suppressive (data not shown), and a significant percentage of the FOXP3+ population produced IFN-γ, IL-2, and IL-10 (Figure 6).
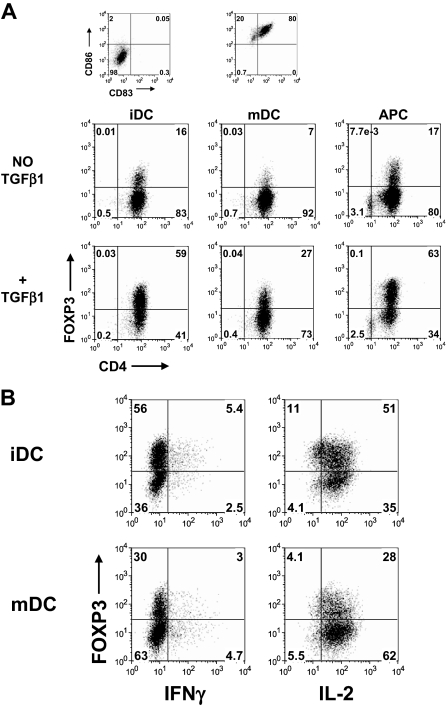
Induction of FOXP3 in CD45RA+ cells activated with antigen-presenting cells required TGFβ but was insufficient to induce T regulatory functions
To determine whether other methods of T cell activation could influence the induction of FOXP3 and T regulatory functions, the CD45RA+ cells were stimulated for 5 days with allogeneic immature dendritic cells (iDC), mature dendritic cells (mDC), or CD3-depleted PBMC (APC) in the presence of 1 μg/mL OKT3 and 50 IU/mL IL-2 with or without 5 ng/mL TGFβ1. Similar to stimulation with anti-CD3 and anti-CD28, TGFβ is required for induction of FOXP3 with iDC and APC (Figure 7A). It is noteworthy that mDC were not very efficient for inducing FOXP3 in CD45RA+ cells. One explanation is that the mDC could be secreting IL-6, which has been shown to inhibit the induction of FOXP3 by TGFβ.22 To test whether the TGFβ-induced FOXP3 expression in CD45RA+ cells stimulated with iDC and mDC was sufficient to suppress IL-2 production, these cells were restimulated with PMA and ionomycin. Similar to activation with anti-CD3 and anti-CD28, both the FOXP3+ and FOXP3− CD45RA+ cells expressed a similar level of IL-2 (Figure 7B).
Figure 7.
Induction of FOXP3 in CD45RA+ cells activated with dendritic cells required TGFβ but was insufficient to suppress IL-2 production. (A) Flow cytometric analyses of FOXP3 with 259D on day 5-activated CD45RA+ cells stimulated with allogeneic monocyte-derived immature dendritic cells (iDC), mature dendritic cells (mDC), or CD3-depleted PBMC (APC) in the presence or absence of TGFβ1. The iDC and mDC were characterized based on their expression of CD86 and CD83. (B) Flow cytometric analyses of FOXP3, IFNγ, and IL-2 on day 7 TGFβ-treated CD45RA+ cells activated with iDC or mDC restimulated with PMA and ionomycin. Data are representative of 3 independent experiments. Numbers in each quadrant as in Figure 1.
Discussion
Expression of Foxp3 has proven to be a reliable marker for mouse thymic-derived nTregs, for Tregs generated in peripheral lymphoid tissues by exposure to antigen under nonstimulatory conditions,23,24 and for those generated in vitro after TCR stimulation in the presence of TGFβ. In contrast, some studies have claimed that in vitro activation of human CD4+CD25− cells by TCR stimulation with anti-CD3 and anti-CD28 results in the induction of FOXP3 and Treg function. We have performed a careful single-cell analysis of the requirements for TCR stimulation and TGFβ costimulation in the induction of FOXP3 in human naive CD4+FOXP3− cells. Human CD4+FOXP3− cells can only be induced to express FOXP3 by TCR stimulation in the presence of TGFβ. FOXP3 expression remains stable in long-term cultures of the induced cells. IL-2, but not TGFβ, is required for maintenance of high levels of FOXP3 expression. One major difference between our studies and those of other groups is that we have used mAb 259D to analyze FOXP3. In our hands, mAb PCH101, the anti-FOXP3 reagent used in many human studies, results in false-positive staining, particularly of activated human CD4+ T cells. We have verified our results with other mAbs to FOXP3, by quantitative PCR analysis, and by siRNA knockdown studies.
After induction of Foxp3 in mouse T cells either by retroviral mediated transfection or TCR stimulation with TGFβ, expression of Foxp3 seems to be sufficient to induce most of the phenotypic and functional characteristics of thymic-derived nTregs. Previous studies25,26 have shown that transfection of human FOXP3 into naive CD4+ T cells can induce Treg functions, although in one study,27 anergy but not suppression was observed. Our results indicate that TGFβ-mediated induction of FOXP3 was insufficient to confer anergy and suppressive function to human CD4+FOXP3− cells.
The TGFβ-induced FOXP3+ cells express both isoforms of FOXP3, up-regulate CTLA-4, and down-regulate CD127. However, the levels of both FOXP3 and CTLA-4 are considerably less than those seen on nTregs that have been stimulated under the same condition. Wan and Flavell have recently described a mutant mouse strain, the nTregs from which express attenuated levels of Foxp3, lack suppressive activities, become Th2 effector cells, but do remain anergic.28 Thus, the absolute levels of Foxp3 expressed by a cell may determine whether it exhibits a T regulatory phenotype. It thus remains possible that the levels of TGFβ-induced FOXP3 are simply too low to drive the T regulatory pathway. Transfection of FOXP3 may result in higher levels and perhaps more stable levels of expression. On the other hand, the levels of FOXP3 on the induced cells are higher than those seen in freshly explanted CD4+CD25hi nTregs that fail to produce cytokines in short-term assays.
Multiple factors may control both the cellular susceptibility to induction of FOXP3 by TGFβ and the capacity of cells that express FOXP3 to manifest T regulatory cell activity. Transfection of FOXP3 was less efficient in reprogramming human CD4+CD45RO+ memory T cell into regulatory cells,25 and Foxp3 could not be induced in recently activated mouse CD4+ T cells by restimulation in the presence of TGFβ.29 Most of our studies were performed with CD4+ T cells from normal adult donors, and it remains possible that the CD45RA+ cells in adult blood are not truly naive and in fact represent memory cells or experienced T cells that cannot respond to or lack components of the FOXP3 driven regulatory pathways. However, similar results were obtained with cord blood CD4+CD25−CD127+CD45RA+ cells. Human CD4+ T cells may also require costimulatory signals or cytokine signals in concert with TGFβ for induction of T regulatory cell function. We have used alternative methods of T cell activation with accessory cells, including CD3-depleted PBMC and monocyte-derived dendritic cells with results similar to those obtained only in the presence of plate-bound antibodies.
Human CD4+CD25− cells may lack critical downstream components of a FOXP3-driven suppressor pathway. It is now clear that interaction of Foxp3 with other transcription factors (NFAT or Runx-1)30,31 or modification of Foxp3 by differential histone acetylation was critical to its action.32 Other studies suggest that induction of some components of the T regulatory phenotype occurs before Foxp3 expression during T-cell development in the thymus and that the function of Foxp3 is to amplify and fix the molecular features of T-regulatory cells.33,34 CD4+Foxp3− cells from the peripheral lymphoid tissues of young mice may retain certain components of the T regulatory pathway that can then be acted on and stabilized by induced Foxp3 to render them Tregs, whereas human CD4+FOXP3− cells may lack these molecules that are essential for the T regulatory phenotype.
One critical question that remains to be addressed is whether FOXP3 can still be considered a definitive marker for human Tregs. FOXP3 expression correlates well with T-regulatory function in CD4+CD25hi cells derived from normal donors. On the other hand, when the immune system has been perturbed, our studies suggest that considerable caution should be exercised before concluding that a FOXP3+ T cell is a bona fide Tregs. It is still unclear whether TGFβ costimulation in vivo can lead to peripheral conversion of human CD4+CD25−FOXP3− to CD4+CD25+FOXP3+ cells. However, TGFβ is found at high levels at inflammatory sites, and some of the CD4+CD25+FOXP3+ cells isolated from such tissues may resemble the FOXP3+ non–T-regulatory cells we have generated in vitro. It has been claimed that Tregs from patients with several autoimmune diseases are deficient in their suppressive capacities in vitro and that this may be one of the fundamental factors that predispose to autoimmune disease.35–40 However, it is possible that some of the CD4+CD25hi cells isolated from patients may also represent TGFβ-induced FOXP3+ non–T-regulatory cells that contaminate the nTreg population and contribute to the defects in suppression that have been observed in these clinical studies. Based on our findings, another concern involves in vitro expansion of human nTregs, which is currently being considered for potential immunotherapy. It remains possible that some of the CD4+CD25+FOXP3+ cells in the starting population are non–T-regulatory cells that had been induced in vivo in response to TGFβ, and these cells may preferentially expand in culture. Although the expanded cells might be all FOXP3+, their functions might be effector instead of regulatory. Likewise, the TGFβ in the serum can potentially induce FOXP3 expression in the few CD4+CD25+FOXP3− cells found in the nTreg starting population and these cells would preferentially outgrow and contaminate the culture.
Finally, antigen-specific TGFβ-induced Foxp3+ murine T cells have proven to be highly effective in the prevention of autoimmune disease. Identification of other factors that might lead to the conversion of human FOXP3− T cells to FOXP3+ T regulatory cells remains an important area for future study. Autoantigen-specific patient-derived Tregs have been proposed for the cellular biotherapy of human autoimmune disease.41 The capacity to convert human autoantigen-specific effectors into Tregs in vitro may ultimately represent a more practical alternative.
Acknowledgments
We thank the NIAID Flow Cytometry Section, particularly Carol Henry, Tom Moyer, and Calvin Eigsti, for all their help in sorting our cells. We also thank Cynthia Matthews and Rosemary Werden in the Department of Transfusion Medicine for providing us with the blood donors. Finally, we greatly appreciated Satya Singh for providing the human cord blood.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
The publisher or recipient acknowledges the right of the US government to retain a nonexclusive, royalty-free license in and to any copyright covering this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.Q.T. initiated and conducted all experiments, prepared the figures, and drafted the manuscript. H.R. helped with the FOXP3 real-time PCR experiments. E.M.S. (principal investigator) supervised the project and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ethan M. Shevach, Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, Bldg 10, Rm 11N315, Bethesda, MD 20892; e-mail: eshevach@niaid.nih.gov.
References
- 1.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 6.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 7.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 9.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25-T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan ME, van Bilsen JH, Bakker AM, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 12.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: A state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 15.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25-T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 18.Weber SE, Harbertson J, Godebu E, et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 19.Fantini MC, Becker C, Tubbe I, et al. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 25.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 26.Oswald-Richter K, Grill SM, Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:e198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 29.Davidson TS, Dipaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Ono M, Yaguchi H, Ohkura N, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–589. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Samanta A, Song X, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 34.Lin W, Haribhai D, Relland LM, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 35.Alvarado-Sánchez B, Hernandez-Castro B, Portales-Perez D, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haas J, Hug A, Viehover A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 38.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–1291. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 41.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells–ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]