Abstract
We recently showed that monoclonal antibodies (mAbs) against β2-microglobulin (β2M) have a remarkably strong apoptotic effect on myeloma cells. The mAbs induced apoptosis by recruiting major histocompatibility complex (MHC) class I to lipid rafts, activated c-Jun N-terminal kinase (JNK), and inhibited phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular signal–regulated kinase (ERK) pathways. Growth and survival cytokines such as interleukin-6 (IL-6) and insulin-like growth factor-I (IGF-I), which could protect myeloma cells from dexamethasone-induced apoptosis, did not affect mAb-mediated cell death. This study was undertaken to elucidate the mechanisms underlying anti-β2M mAb–induced PI3K/Akt and ERK inhibition and the inability of IL-6 and IGF-I to protect myeloma cells from mAb-induced apoptosis. We focused on lipid rafts and confirmed that these membrane microdomains are required for IL-6 and IGF-I signaling. By recruiting MHC class I into lipid rafts, anti-β2M mAbs excluded IL-6 and IGF-I receptors and their substrates from the rafts. The mAbs not only redistributed the receptors in cell membrane, but also abrogated IL-6– or IGF-I–mediated Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), PI3K/Akt, and Ras/Raf/ERK pathway signaling, which are otherwise constitutively activated in myeloma cells. Thus, this study further defines the tumoricidal mechanism of the mAbs and provides strong evidence to support the potential of these mAbs as therapeutic agents for myeloma.
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow.1,2 Binding of myeloma cells to bone marrow stromal cells triggers transcription and secretion of cytokines from stromal cells, which not only promote growth, survival, and migration of myeloma cells but also confer resistance to conventional chemotherapy.1–4 Previous studies have shown that cytokines such interleukin-6 (IL-6) and insulin-like growth factor-I (IGF-I) are the major growth and survival factors for myeloma cells,5–8 and play a crucial role in the onset of plasma cell tumors in mice.9 Specifically, IL-6 binds to glycoprotein (gp) 80 (CD80; IL-6 receptor [IL-6R]), which is expressed on most myeloma cell lines and patient tumors, and induces phosphorylation and dimerization of gp130. Phosphorylation of gp130 in turn activates multiple downstream signaling pathways, such as Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3),10 Ras/Raf/mitogen-activated protein kinases (MAPKs),11 and phosphatidylinositol 3-kinase (PI3K)/Akt,12 and triggers myeloma cell growth, survival, and drug resistance. Likewise, IGF-I binds to IGF-I receptor (IGF-IR) and exerts its antiapoptotic effects on myeloma cells via activating antiapoptotic signaling pathways, such as Ras/Raf/MAPK and PI3K/Akt.13,14 Therefore, it may be useful to disrupt growth factor–mediated antiapoptotic signaling pathways for myeloma therapy, which might provide the framework to develop and validate novel antimyeloma agents to overcome drug resistance and improve patient outcome.
Lipid rafts, cholesterol- and glycosphingolipid-enriched dynamic patches in the plasma membrane, organize the plasma membrane into functional units.15 These raft domains act as platforms for conducting different signals into cells for various functions, including cytokine-mediated growth signaling.16 Integral proteins in the cellular membrane, such as caveolins and flotillins, can modify lipid rafts structurally and functionally, and may therefore affect subsequent cellular functions.17,18 Some reports have shown that growth factors, such as IL-6, induce translocation of their receptors to lipid rafts and confer protection against dexamethasone treatment.19,20 Remacle-Bonnet and coworkers21 observed that lipid rafts segregated proapoptotic from antiapoptotic IGF-IR–mediated signaling in tumor cells, suggesting that the localization of growth factor receptors outside lipid rafts might be involved in the transduction of apoptotic signals. Furthermore, we and others demonstrated that lipid rafts might be involved in anti–β2-microglobulin (β2M), major histocompatibility complex (MHC) class II, and CD20 monoclonal antibody (mAb)–induced apoptosis in tumor cells,22–25 indicating that lipid rafts might also be an important platform for the mAb-mediated tumoricidal effects on myeloma cells.
We have recently shown that anti-β2M mAbs have remarkable tumoricidal activity on myeloma cells both in vitro and in xenograft myeloma severe combined immunodeficiency (SCID) mouse models.25 We demonstrated that anti-β2M mAbs induced myeloma cell apoptosis by recruiting MHC class I molecules to lipid rafts, activated c-Jun N-terminal kinase (JNK) and inhibited PI3K/Akt and ERK, compromised mitochondrial integrity, and activated the caspase-9–dependent cascade. To further elucidate the mechanisms of mAb-induced inhibition of PI3K/Akt- and ERK-signaling pathways and the inability of IL-6 and IGF-I to protect myeloma cells from apoptosis, we examined the localization of cytokine receptors and their signaling pathways in myeloma cells with or without treatment with anti-β2M mAbs. We confirmed that IL-6– and IGF-I–signaling pathways depend on lipid rafts, and showed that anti-β2M mAbs recruit MHC class I to and exclude cytokine receptors from lipid rafts.
Patients, materials, and methods
Myeloma cell lines, primary myeloma cells, and reagents
The human myeloma cell line ARP-1 was established at the Arkansas Cancer Research Center (Little Rock, AR) from bone marrow aspirates of patients with MM, and MM.1S was kindly provided by Dr Steven Rosen of Northwestern University (Chicago, IL). Other cell lines were purchased from American Type Culture Collection (Rockville, MD). All cell lines were cultured in RPMI-1640 medium containing 10% (vol/vol) heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in humidified 95% air and 5% CO2. Primary myeloma cells were isolated from bone marrow aspirates obtained from patients during a routine clinic visit. CD138++ myeloma cells were isolated by magnetic-bead sorting (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The study was approved by the Institutional Review Board at The University of Texas M. D. Anderson Cancer Center. Informed consent was obtained in accordance with the Declaration of Helsinki.
Recombinant human IL-6 and IGF-I were purchased from R&D Systems (Minneapolis, MN). Mouse IgG1 and dexamethasone were purchased from Sigma (St Louis, MO). Monoclonal antibodies against HLA-ABC (clone W6/32) were purchased from Serotec (Raleigh, NC).
Generation of β2M-specific mAbs
We generated a panel of β2M-specific mAbs as previously described.25 Among of them, D1 and E6 (isotype IgG1) were chosen for this study because of their strong antimyeloma activities.
Apoptosis assays
Cells were incubated with 50 μg/mL of the β2M-specific mAbs D1 or E6, or mouse IgG1 as IgG control, with or without the addition of IL-6 (10 ng/mL) or IGF-I (50 ng/mL) for 48 hours. In some experiments, cells were treated with dexamethasone (10 μM) in the presence of β2M-specific mAbs and IL-6 (10 ng/mL) for 48 hours. The fraction of apoptotic cells was determined by staining cells suspended in Annexin-V–binding buffer (PharMingen, San Diego, CA) with FITC-conjugated Annexin-V and propidium iodide (PI), according to manufacturer's instructions. After 15 minutes of incubation at room temperature, samples were analyzed by flow cytometry. Apoptotic cells were determined as Annexin-V+ cells.
RNA interference
Double-stranded, 21-mer small interfering RNA (siRNA) corresponding to β2M was designed with the following sense and antisense sequences. Sense: 5′-GAUUCAGGUUUACUCAC GUdTdT-3′; and antisense: 5′-ACGUGAGUAAACCUGAAUCdTdT-3′, starting from nucleotide 91 of the β2M sequence (GenBank accession number AB02128826). The siRNA was synthesized by Dharmacon (Lafayette, CO). Cells were harvested, plated on a 24-well plate with 2 × 105 cells per well, and transiently transfected 24 hours later with specific β2M siRNA or nonspecific/control siRNA using the Oligofectamine transfection reagent (Mirus, Madison, WI) according to manufacturer's instructions. At 72 hours after the transfection, cells were harvested to examine surface β2M and HLA-ABC protein expression or used for experiments.
Isolation of lipid rafts by sucrose density gradient ultracentrifugation
The Caveolae/Raft Isolation Kit (Sigma) was used to isolate lipid rafts as low-density, detergent-resistant membrane fractions by sucrose density gradient centrifugation using 1% Triton X-100. Briefly, 5 × 107 cells were lysed for 30 minutes in ice-cold lysis buffer. Cell lysates were mixed with OptiPrep (Sigma) to 35%, placed at the bottom of the ultracentrifuge tube, overlaid with 4 layers of 30% to 0% OptiPrep, and centrifuged at 200 000g using a TFT 65.13 rotor (Kontron Instruments, Milan, Italy) for 4 hours at 4°C. A total of 9 fractions (1 mL each) were collected from the top to the bottom of the gradients. The lipid rafts determined with cholera toxin B subunit (CTB) binding for GM1 gangliosides and with a caveolin-1–specific antibody were found in fractions 2 to 5. Nonlipid raft fractions were present in fractions 7 to 9, which were negatively stained by GM1 gangliosides and caveolin-1.
Cholesterol depletion
For cholesterol depletion, myeloma cells were preincubated with 5 mM methyl-β-cyclodextrin (MCD; Sigma) for 30 minutes, washed, and incubated with or without β2M-specific mAbs (50 μg/mL) for 48 hours, followed by cell apoptosis analysis.
Immunoprecipitation assay
Myeloma cells were incubated with IL-6 (10 ng/mL) or IGF-I (50 ng/mL) with or without 50 μg/mL of β2M-specific mAbs or mouse IgG1 on ice for 30 minutes, washed, and lysed in 1 mL RIPA buffer (10 mM Tris-HCL buffer [pH 7.5], 1% NP-40, 0.25% deoxycholate [wt/vol], 2 mM EDTA, and 10 mM orthovanadate). Cell lysates were incubated with antibodies specific to caveolin-1, gp130, or IGF-IRβ, followed by precipitating with protein G–Sepharose in a 50% wt/vol slurry. Immunoprecipitated proteins were washed in RIPA buffer, subjected to SDS-PAGE, and immunoblotted with specific antibodies against caveolin-1, gp130, IGF-IRβ, or MHC class I molecules.
Western blotting analysis
Cells were cultured with IL-6 (10 ng/mL) or IGF-I (50 ng/mL) with or without 50 μg/mL of β2M-specific mAbs, harvested, washed, and lysed with lysis buffer (50 mM Tris [pH 7.5], 140 mM NaCl, 5 mM EDTA, 5 mM NaN3, 1% Triton X-100, 1% NP-40, and 1 × protease inhibitor cocktail). Cell lysates were subjected to SDS-PAGE, transferred to polyvinylidene difluoride membrane, and immunoblotted with antibodies against phosphorylated or nonphosphorylated IL-6R, JAK, STAT3, Akt, Raf, ERK1/2, IGF-IRβ, IRS-1, ASK1, MLK3, MAPK kinase kinase-1 (MEKK1), MKK4, MKK7, and JNK (Cell Signaling Technology, Beverly, MA; and Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies conjugated to horseradish peroxidase were used for detection, followed by enhanced chemiluminescence (Pierce, Rockford, IL) and autoradiography. For protein quantification, blots were scanned and analyzed by spot densitometry, and results were expressed as average value of pixels enclosed (AVG), calculated as the sum of all the pixel values after background correction divided by area.
Statistical analysis
All data are shown as means (± SD). The Student t test was used to compare various experimental groups. Significance was set at a P value less than .05.
Results
Growth and survival factors do not abrogate β2M-specific mAb–induced myeloma cell apoptosis
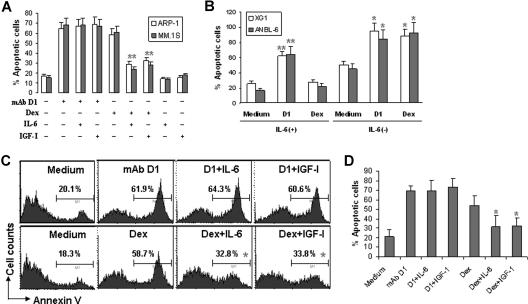
Previous studies showed that addition of IL-6 protected myeloma cells from dexamethasone-induced apoptosis. As we previously reported that anti-β2M mAbs induced apoptosis in myeloma cells, we asked whether IL-6 would also protect myeloma cells from the mAb-induced apoptosis. In our experiments, β2M-specific mAbs (50 μg/mL) were added to cultures of the myeloma cell lines ARP-1 and MM.1S, with or without addition of 10 ng/mL of human IL-6. Cell apoptosis was examined 48 hours later by Annexin-V staining assay. As shown in Figure 1A, β2M-specific mAb D1, but not mouse IgG1 (data not shown), and dexamethasone effectively induced apoptosis in myeloma cells. The addition of IL-6 did not affect mAb-induced apoptosis but significantly undermined dexamethasone-induced cell death (P < .05; Figure 1A). Increasing IL-6 concentration to 100 ng/mL still failed to protect myeloma cells from β2M-specific mAb–induced apoptosis (data not shown). Likewise, β2M-specific mAbs (P < .01; compared with medium controls) but not dexamethasone killed the IL-6–dependent cell lines XG1 and ANBL-6 in their culture with the addition of exogenous IL-6 (Figure 1B; 2 ng/mL for normal culture, or 20 ng/mL for high concentration; data not shown). However, in the absence of IL-6, both the mAb and dexamethasone induced apoptosis in these 2 cell lines (P < .05; compared with medium controls).
Figure 1.
Effects of IL-6 and IGF-I on β2M-specific mAb– or dexamethasone-induced apoptosis in myeloma cells. (A) Apoptosis of ARP-1 and MM.1S myeloma cells in 48-hour cultures with β2M-specific mAb D1 (50 μg/mL) or dexamethasone (10 μM) in the presence or absence of IL-6 (10 ng/mL) or IGF-I (50 ng/mL). Similar results were obtained with other myeloma cell lines. (B) Apoptosis of 2 IL-6–dependent cell lines XG1 and ANBL-6 in 24-hour cultures with β2M-specific mAb D1 (50 μg/mL) or dexamethasone (10 μM) in the presence or absence of IL-6 (10 ng/mL). Flow cytometry analysis from (C) a representative patient with myeloma and (D) pooled data from all patients showing apoptosis of primary myeloma cells in 24-hour cultures with β2M-specific mAb D1 (50 μg/mL) or dexamethasone (10 μM) in the presence or absence of IL-6 (10 ng/mL) or IGF-I (50 ng/mL). Similar results were obtained with primary myeloma cells with anti-β2M mAb E6. Apoptotic cells were determined by Annexin-V–binding assay. Results of 3 experiments are shown. Error bars indicate SD. *P < .05; **P < .01.
IGF-I is another important growth and survival factor for myeloma cells. Therefore, we examined the effect of IGF-I on β2M-specific mAb–induced apoptosis. Myeloma cells were cultured with the β2M-specific mAbs with or without the addition of IGF-I (50 ng/mL). Again, results showed that IGF-I did not affect β2M-specific mAb–induced cell death but reduced dexamethasone-mediated apoptosis in myeloma cells (Figure 1A).
We next investigated the activity of IL-6 and IGF-I on freshly isolated primary tumor cells from patients with MM. Purified myeloma cells obtained from bone marrow aspirates of 4 newly diagnosed, previously untreated patients with MM were examined and showed sensitivity to β2M-specific mAb–mediated killing (data not shown). As shown by the representative results depicted in Figure 1C, addition of 10 ng/mL IL-6 or 50 ng/mL IGF-I to the cultures had no effect on β2M-specific mAb–induced apoptosis in the primary myeloma cells, whereas both IL-6 and IGF-I significantly reduced the percentages of apoptotic cells induced by dexamethasone (P < .05). Similar results were obtained with primary myeloma cells from all 4 patients (Figure 1D). Taken together, these results indicate that although IL-6 and IGF-I are potent protectors of myeloma cells against dexamethasone, they could not abrogate mAb-mediated apoptotic activities in myeloma cells.
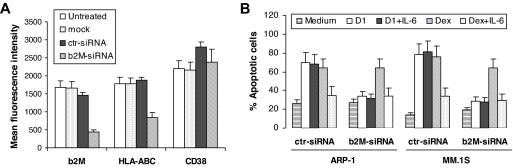
To confirm the importance of surface β2M as the target for β2M-specific mAb–induced apoptosis but not for dexamethasone-induced apoptosis, and to exclude the involvement of surface β2M in IL-6–mediated protection of apoptosis in myeloma cells, siRNA specific for human β2M gene was synthesized and used to knock down the β2M expression in myeloma cells. We were able to achieve 70% to 80% reduction of surface β2M and HLA-ABC protein expression on myeloma cells on day 3 after transfection with β2M-specific siRNA but not with control siRNA or mock transfection (Figure 2A). Detection of β2M mRNA by reverse transcription–polymerase chain reaction (RT-PCR) confirmed these results (data not shown). This treatment was specific for β2M, as surface expression of HLA-DR (data not shown) and CD38 remained unchanged. Knock-down of surface β2M/MHC class I on myeloma cells rendered cells resistant to β2M-specific mAb–induced apoptosis, but did not affect dexamethasone-induced or IL-6–mediated protection of myeloma cell apoptosis (Figure 2B).
Figure 2.
Knock-down of surface β2M/MHC class I abrogates β2M-specific mAb– but not dexamethasone-induced apoptosis in myeloma cells. (A) Levels (mean fluorescence intensity) of surface β2M, HLA-ABC and CD38 on untreated myeloma cells and on cells treated with mock transfection or transfected with control (ctr) siRNA or β2M-specific siRNA. Shown are the results of the MM.1S myeloma cell line. Similar results were obtained from ARP-1. Analysis was performed 72 hours after transfection. (B) Apoptosis of myeloma cells (ARP-1 and MM.1S) transfected with control (ctr) siRNA or β2M-siRNA in cultures with β2M-specific mAb D1 (50 μg/mL) or dexamethasone (10 μM) in the presence or absence of IL-6 (10 ng/mL). In these experiments, cells were transfected with 400 nM siRNA, and 72 hours later, washed and incubated with β2M-specific mAbs or dexamethasone for another 48 hours. Apoptosis was detected by Annexin-V–binding assay. Results of 4 experiments performed are shown. Similar results were obtained from other myeloma cell lines and anti-β2M mAb E6. Error bars indicate SD.
β2M-specific mAbs exclude growth and survival factor receptors from lipid rafts
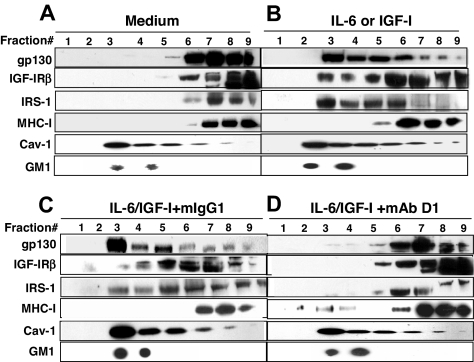
Our previous study showed that β2M-specific mAbs inhibited MAPK kinase (MEK)/ERK and PI3K/Akt pathways by binding to β2M and recruiting MHC class I to the lipid rafts. As these pathways are downstream of IL-6 and IGF-I receptor activation, we hypothesized that MHC class I relocation to lipid rafts may disrupt IL-6 and IGF-I receptor signaling, as lipid rafts are considered to function in part as platforms for signaling from the receptors. Therefore, we examined the localization of IL-6 and IGF-I receptors on myeloma cells before and after β2M-specific mAb treatment. Myeloma cells were incubated with IL-6 (10 ng/mL) or IGF-I (50 ng/mL), with or without β2M-specific mAbs (50 μg/mL). Mouse IgG1 was used as control for the mAbs. Cells without treatment were used as control. After 30 minutes of treatment, cell lysates were prepared and separated using a discontinuous sucrose gradient ultracentrifugation followed by immunoblotting with specific antibodies. In the light buoyant density fractions, fractions 2 to 5 were positive for GM1 gangliosides, identified by CTB binding, and contain lipid rafts, which stained positive for caveolin-1, a raft-associated protein absent in the nonraft fractions 7 to 9 (Figure 3). As shown in Figure 3A, IL-6R gp130, IGF-IRβ, IRS-1 (an IGF-IR substrate), and MHC class I were detected in the nonraft fractions in control myeloma cells. Upon IL-6 or IGF-I stimulation, the majority of gp130, IGF-IRβ, and IRS-1 molecules were detected in the raft fractions, whereas MHC class I molecules were located in the nonraft fractions, indicating that the cytokine receptors and substrates were relocalized to lipid rafts for signaling (Figure 3B). Surprisingly, in cells treated with both anti-β2M mAb (D1) and IL-6 or IGF-I (Figure 3D), but not mouse IgG1 and IL-6 or IGF-I (Figure 3C), most gp130 and IGF-IRβ were detected in the nonlipid raft fractions and fewer IRS-1 molecules were present in the raft fractions, while MHC class I molecules were recruited to the lipid raft fractions as we showed previously. These findings suggest that the cytokine receptors and their substrate were excluded from the lipid rafts as a result of β2M-specific mAb–mediated recruitment of MHC class I to the rafts.
Figure 3.
β2M-specific mAbs exclude growth factor receptors and their substrates from lipid rafts. Shown is the localization of IL-6R gp130, IGF-IRβ, IRS-1, MHC class I, caveolin-1 (Cav-1), and GM1 gangliosides in lipid rafts (fractions 2–5) or nonraft fractions (fractions 7–9) in (A) untreated myeloma cells, (B) IL-6– or IGF-I–activated myeloma cells, (C) IL-6– or IGF-I–activated myeloma cells in the presence of mouse IgG1, and (D) IL-6– or IGF-I–activated myeloma cells in the presence of anti-β2M mAb D1. The concentrations of IL-6 (10 ng/mL), IGF-I (50 ng/mL), mouse IgG1 (50 μg/mL), and anti-β2M mAb D1 (50 μg/mL) were used. Lipid rafts were isolated from myeloma cells after treatment. The raft fractions were confirmed by positive staining for GM1 gangliosides and identified by CTB binding and by antibody specific to caveolin-1, a raft-associated protein. Results obtained with D1 mAb on MM.1S myeloma cells from 1 representative experiment of 4 performed are shown. Similar results are obtained with other tumor cell lines, and with anti-β2M mAb E6.
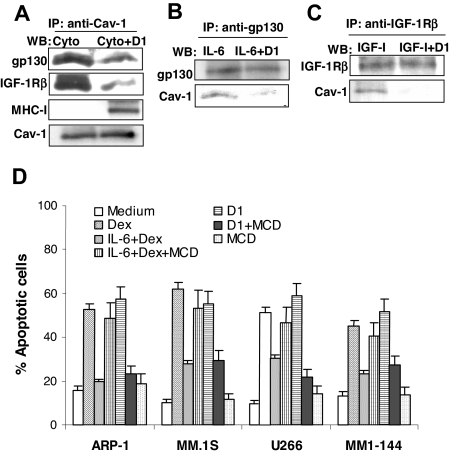
To confirm these results, an immunoprecipitation assay was used to analyze the interactions of MHC class I, gp130, or IGF-IRβ with caveolin-1, a raft-associated protein able to directly bind cholesterol. Cell lysates prepared from myeloma cells treated with IL-6 or IGF-I, with or without β2M-specific mAbs, were precipitated by a specific antibody against caveolin-1, followed by Western blotting analysis to detect protein expression of gp130, IGF-IRβ, and MHC class I by specific antibodies. As shown in Figure 4A, MHC class I and caveolin-1 were coprecipitated from cells treated with cytokine and β2M-specific mAbs but not from cells treated with cytokine alone, confirming that MHC class I molecules were localized within the lipid rafts upon β2M-specific mAb treatment. In contrast, both gp130 and IGF-IRβ were strongly associated with caveolin-1 in cells treated with the cytokine (IL-6 or IGF-I), and the addition of β2M-specific mAbs significantly reduced the amounts of gp130 and IGF-IRβ coprecipitated with caveolin-1. Likewise, immunoprecipitation using antibodies specific to gp130 (Figure 4B) or IGF-IRβ (Figure 4C) yielded similar amounts of gp130 or IGF-IRβ from cells treated with the cytokine (IL-6 or IGF-I) without or with β2M-specific mAbs, whereas the amounts of coprecipitated caveolin-1 were significantly lower in cells treated with the cytokine and the mAbs than cells treated with the cytokine alone. These results indicate that gp130 and IGF-IRβ were physically associated with caveolin-1 in cytokine-treated cells, but not at all or much less physically associated in cytokine- and β2M-specific mAb–treated myeloma cells.
Figure 4.
Association of growth factor receptors with and integrity of lipid rafts in myeloma cell apoptosis. Immunoprecipitation (IP) using antibody specific to (A) caveolin-1 (Cav-1), (B) IL-6R gp130, and (C) IGF-IRβ in myeloma cells treated with cytokines (Cyto; IL-6 or IGF-I) or cytokines together with mAb D1 (Cyto + D1), followed by Western blotting analysis (WB) using antibodies against gp130, IGF-IRβ, MHC class I (W6/32), or caveolin-1. (A-C) Expression of caveolin-1, gp130, and IGF-IRβ, respectively, serve as loading controls. Results obtained with D1 mAb on MM.1S from 1 representative experiment of 4 performed are shown. Similar results were obtained with other tumor cell lines. (D) To deplete cholesterol and disrupt lipid rafts, cells were preincubated with MCD (5 mM; titrated in preliminary experiments) for 30 minutes, washed, and incubated further with β2M-specific mAb D1 (50 μg/mL) or dexamethasone (10 μM) in the presence or absence of IL-6 (10 ng/mL). Percentage of apoptotic cells was measured at 48 hours by Annexin-V–binding assay. Results from 4 experiments performed are shown. Similar results were obtained with anti-β2M mAb E6. Error bars indicate SD.
Disruption of lipid rafts abrogates both IL-6 signaling and β2M-specific mAb apoptotic effects in myeloma cells
To confirm the importance of the lipid rafts in IL-6 signaling and protection of myeloma cell apoptosis, we treated myeloma cells with MCD, an agent that disrupts the structure of lipid rafts in cell membrane. As shown in Figure 4D, MCD treatment abrogated IL-6–mediated protection of myeloma cell apoptosis induced by dexamethasone (P < .05 to P < .01), indicating that lipid rafts are crucial for IL-6R signaling to activate antiapoptotic pathways in myeloma cells. As expected, MCD also abrogated β2M-specific mAb–induced apoptosis in myeloma cells. Taken together, these results demonstrate that the β2M-specific mAbs induced dominant changes in the distribution of MHC I molecules and cytokine receptors in lipid rafts.
β2M-specific mAbs inhibit growth and survival factor–mediated antiapoptotic signaling pathways
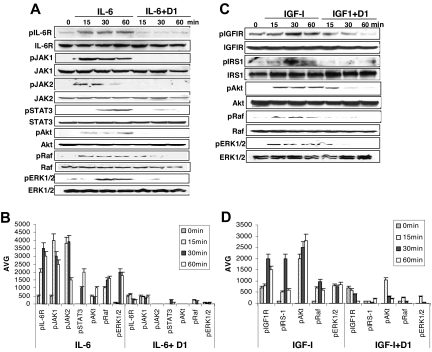
To further elucidate the molecular mechanisms underlying apoptosis protection, we next examined the impact of β2M-specific mAbs on the downstream signaling pathways of cytokine receptors. First, we focused on IL-6–activated JAK/STAT3, PI3K/Akt, and Ras/Raf/ERK pathways, which are essential to myeloma cell growth and survival. Myeloma cells were treated with IL-6 without or with β2M-specific mAbs or mouse IgG1. Western blotting analysis was performed using specific antibodies against phosphorylated or nonphosphorylated IL-6R, JAK1/1, STAT3, Akt, Raf, and ERK1/2. As shown in Figure 5A, treatment of cells with IL-6 or IL-6 plus mouse IgG1 (data not shown) induced phosphorylation of IL-6R (pIL-6R), and up-regulated the levels of pJAK1/2, pSTAT3, pAkt, pRaf, and pERK, indicating that IL-6 activated JAK/STAT3, PI3K/Akt, and Ras/Raf/ERK signaling pathways. In contrast, β2M-specific mAb treatment inhibited IL-6–induced phosphorylation of IL-6R and the kinases. These results are supported by protein quantification data of phosphorylated IL-6R and kinases (P < .05 and P < .01; Figure 5B[b]). The levels of nonphosphorylated IL-6R and kinases remained unchanged. After 60 minutes of treatment, the kinase activities were undetectable in β2M-specific mAb–treated myeloma cells.
Figure 5.
β2M-specific mAbs abrogate IL-6- and IGF-I-induced signaling pathways. (A) Western blot analysis and (B) densitometric data (AVG) showing protein levels of phosphorylated (p) and nonphosphorylated IL-6R, JAK1, JAK2, STAT3, Akt, Raf, and ERK1/2 in myeloma cells treated with IL-6 (10 ng/mL) or IL-6 together with β2M-specific mAbs (50 μg/mL). (C) Western blot analysis and (D) densitometric data (AVG) showing protein levels of phosphorylated (p) and nonphosphorylated IGF-IRβ, IRS-1, Akt, Raf, and ERK1/2 in myeloma cells treated with IGF-I (50 ng/mL) or IGF-I together with β2M-specific mAbs (50 μg/mL). Results obtained with D1 mAb on MM.1S from 1 representative experiment of 3 performed are shown. Similar results were obtained with other tumor cell lines. Error bars indicate SD.
Second, we examined the impact of β2M-specific mAbs on IGF-I–mediated signaling pathways, including PI3K/Akt and Ras/Raf/ERK. As shown in Figure 5C, IGF-I or IGF-I plus mouse IgG1 (data not shown) stimulated the phosphorylation of IGF-IRβ and its substrate IRS-1 and, as a consequence, up-regulated the expression of pAkt and pERK in myeloma cells. Treatment of cells with β2M-specific mAbs significantly down-regulated the levels of pIGF-IRβ, pIRS-1, pAkt, and pERK induced by IGF-I (protein quantification data shown in Figure 5D; P < .05 and P < .01). These results indicated that β2M-specific mAbs abrogate growth factor–mediated antiapoptotic signaling pathways in myeloma cells.
β2M-specific mAbs activate the upstream kinases of the JNK signaling pathway
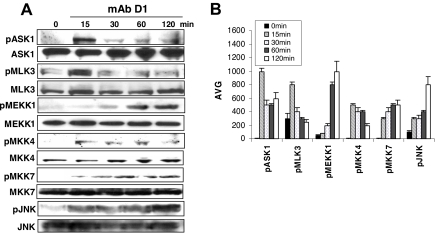
Since our previous studies demonstrated that β2M-specific mAbs induced myeloma cell apoptosis via JNK activation, we examined the activities of kinases upstream of the JNK signaling pathway, including ASK1, MLK3, MEKK1, and MKK4/7. As shown in Figure 6A,B, treatment of myeloma cells with β2M-specific mAbs significantly increased the level of phosphorylated ASK1, MLK3, MEKK1, MKK4, and MKK7 (P < .01). The phosphorylation of these kinases was observed as early as 15 minutes and lasted for 2 hours after the treatment, although their kinetics were slightly different. As a consequence, protein levels of pJNK were increased in β2M-specific mAb–treated myeloma cells. These results further confirm that β2M-specific mAbs activate the JNK signaling pathway.
Figure 6.
β2M-specific mAbs activate the JNK signaling pathway. (A) Western blot analysis and (B) densitometric data (AVG) showing protein levels of phosphorylated (p) and nonphosphorylated ASK1, MLK3, MEKK1, MKK4, MKK7, and JNK in β2M-specific mAb (D1)–treated myeloma cells. Results obtained with D1 mAb on MM.1S myeloma cells from 1 representative experiment of 3 performed are shown. Similar results were obtained with β2M-specific mAb E6 with this and other myeloma cell lines. Error bars indicate SD.
Discussion
The importance of IL-6 and IGF-I in the pathogenesis of MM is well documented.5–8 Recent studies have also shown that these cytokines play an important role in myeloma cell survival and protect the tumor cells from chemotherapy drugs, such as dexamethasone-induced apoptosis.19 IL-6 binds to its receptor gp80, induces phosphorylation and dimerization of gp130, and activates JAK/STAT3, PI3K/Akt, and Ras/Raf/ERK pathways in myeloma cells.10–12 STAT3 regulates downstream protein expression of Bcl-2 family members Bcl-XL and Mcl-1, which inhibits mitochondria-dependent caspase cascade activation.27,28 Other studies showed that IL-6 protects myeloma cells against dexamethasone-induced apoptosis via activating the PI3K/Akt-signaling pathway.29 Likewise, IGF-I is another important growth and survival factor for myeloma cells. The assembly of the signaling complex at the cytoplasmic domain of IGF-IR results in the activation of PI3K/Akt and Ras-dependent MAPK cascades.13,14 The strong antiapoptotic activity of IGF-I in myeloma cells is mediated through Akt-mediated inactivation of the proapoptotic Bcl-2 family member Bad.30 In this study, we showed that, although IL-6 and IGF-I abrogate dexamethasone-mediated apoptosis, high levels of the cytokines could not reduce the apoptotic effects of β2M-specific mAbs on myeloma cells.
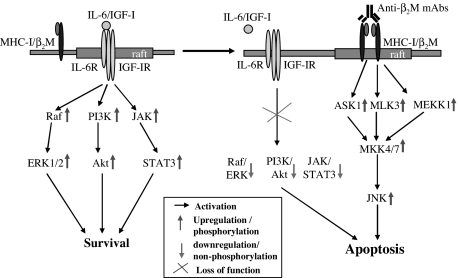
In our previous study, we demonstrated that by relocating to lipid rafts, MHC class I recruits and activates Lyn and PLCγ2, which in turn activate JNK. In this study, we examined the activation and phosphorylation of the kinases upstream of JNK and showed that ASK1, MLK3, MEKK1, MKK4, and MKK7 are indeed phosphorylated in myeloma cells following anti-β2M mAb treatment. Thus, these results confirm our previous observations and conclusions. As shown in Figure 7, Lyn and PLCγ2 activation led to phosphorylation of ASK1, MLK3, and MEKK1, which in turn phosphorylate MKK4/7. As a consequence, MKK4/7 phosphorylates and activates JNK, leading to myeloma cell apoptosis. However, these findings do not explain why PI3K/Akt and ERK pathways were inhibited by β2M-specific mAbs.
Figure 7.
Schematic presentation of IL-6, IGF-I, and β2M-specific mAb–induced antiapoptotic or apoptotic signaling pathways in myeloma cells. Raft indicates lipid rafts.
To further define the mechanisms underlying β2M-specific mAb–induced apoptosis and the inability of IL-6 and IGF-I to counteract the effects of the mAbs on myeloma cells, we focused on lipid rafts because they are involved in cell growth and apoptosis signaling.15,16 IL-6 binds to IL-6R, recruits the receptors to lipid rafts, and stimulates downstream antiapoptotic signaling pathways to resist dexamethasone treatment. In this study, we confirmed that stimulation of myeloma cells by IL-6 or IGF-I led to relocation of gp130 or IGF-IRβ, and its substrate IRS-1, to lipid rafts and increased affinity of receptor binding to caveolin-1, which regulates the structure and function of lipid rafts. Disruption of the integrity of lipid rafts interrupted IL-6 and IGF-I signaling and abrogated cytokine-mediated protection of myeloma cells against dexamethasone-induced apoptosis. In addition, we recently showed that MHC class I molecules are not present in lipid rafts under physiologic conditions in myeloma cells. However, β2M-specific mAbs bound to surface β2M/MHC class I molecules and recruited them to lipid rafts, leading to MHC class I binding to caveolin-1 and consequently activating the upstream kinases of JNK. Moreover, we observed that MHC class I molecules replace gp130, IGF-IRβ, and IRS-1 in lipid rafts, because these receptors and the substrates were found in the nonraft fractions after β2M-specific mAb treatment. These findings indicate that β2M-specific mAbs exclude growth factor receptors from lipid rafts and abrogate IL-6– or IGF-I–mediated JAK/STAT3, PI3K/Akt, and Ras/Raf/ERK pathway signaling, which are otherwise constitutively activated in myeloma cells. Thus, these results provide a plausible explanation for anti-β2M mAb–induced inhibition of PI3K/Akt and ERK pathways in myeloma cells. Our results also showed that disruption of lipid rafts by MCD abrogates both β2M-specific mAb–induced apoptotic effects on myeloma cells and IL-6–mediated protection against dexamethasone treatment, indicating that the integrity of lipid rafts is important for both proapoptotic and antiapoptotic signaling.
In conclusion, this study demonstrated that anti-β2M mAbs induce cell death via recruiting MHC class I molecules to lipid rafts, which not only activate JNK via Lyn and PLCγ2, but also inhibit PI3K/Akt and ERK pathways by excluding IL-6 and IGF-I receptors from lipid rafts and disrupting their signaling pathways (Figure 7). These findings explain why the cytokines protect myeloma cells from dexamethasone-induced apoptosis but had no effect on cell death induced by the mAbs. Thus, this study further defines the tumoricidal mechanism of the mAbs and provides strong evidence to support the potential and implication of these mAbs as therapeutic agents for myeloma.
Acknowledgments
We thank Ms Alison Woo for providing editorial assistance.
This work was supported by National Cancer Institute grants (R01 CA96569 and R01 CA103978), the Leukemia and Lymphoma Society Translational Research Grant (6041–03), Multiple Myeloma Research Foundation (29–05), and Commonwealth Foundation for Cancer Research.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.Y. and Q.Y. initiated the work, designed the experiments, and wrote the paper. J.Y., X.Z., J.W., J.Q., and L.Z. performed the experiments and statistical analyses. M.W. and L.W.K. provided patient samples and critical suggestions to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qing Yi, Department of Lymphoma and Myeloma, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0903, Houston, TX 77030; e-mail: qyi@mdanderson.org.
References
- 1.Anderson K. Advances in the biology of multiple myeloma: therapeutic applications. Semin Oncol. 1999;26:10–22. [PubMed] [Google Scholar]
- 2.Anderson KC. Multiple myeloma: advances in disease biology: therapeutic implications. Semin Hematol. 2001;38:6–10. doi: 10.1016/s0037-1963(01)90088-5. [DOI] [PubMed] [Google Scholar]
- 3.Epstein J, Yaccoby S. Consequences of interactions between the bone marrow stroma and myeloma. Hematol J. 2003;4:310–314. doi: 10.1038/sj.thj.6200313. [DOI] [PubMed] [Google Scholar]
- 4.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 6.Abroun S, Ishikawa H, Tsuyama N, et al. Receptor synergy of interleukin-6 (IL-6) and insulin-like growth factor-I in myeloma cells that highly express IL-6 receptor alpha [corrected]. Blood. 2004;103:2291–2298. doi: 10.1182/blood-2003-07-2187. [DOI] [PubMed] [Google Scholar]
- 7.Freund GG, Kulas DT, Mooney RA. Insulin and IGF-1 increase mitogenesis and glucose metabolism in the multiple myeloma cell line, RPMI 8226. J Immunol. 1993;151:1811–1820. [PubMed] [Google Scholar]
- 8.Frostad S, Bjerknes R, Hervig T, Nesthus I, Olweus J, Bruserud OO. Malignancy: insulin-like growth factor-1 (IGF-1) is a costimulator of the expansion of lineage committed cells derived from peripheral blood mobilized CD34+ cells in multiple myeloma patients. Hematol. 1999;4:217–229. doi: 10.1080/10245332.1999.11746445. [DOI] [PubMed] [Google Scholar]
- 9.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–872. [PubMed] [Google Scholar]
- 10.Klein B. Update of gp130 cytokines in multiple myeloma. Curr Opin Hematol. 1998;5:186–191. doi: 10.1097/00062752-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Lentzsch S, Chatterjee M, Gries M, et al. PI3-K/AKT/FKHR and MAPK signaling cascades are redundantly stimulated by a variety of cytokines and contribute independently to proliferation and survival of multiple myeloma cells. Leukemia. 2004;18:1883–1890. doi: 10.1038/sj.leu.2403486. [DOI] [PubMed] [Google Scholar]
- 12.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 13.Qiang YW, Kopantzev E, Rudikoff S. Insulinlike growth factor-I signaling in multiple myeloma: downstream elements, functional correlates, and pathway cross-talk. Blood. 2002;99:4138–4146. doi: 10.1182/blood.v99.11.4138. [DOI] [PubMed] [Google Scholar]
- 14.Menu E, Kooijman R, Van Valckenborgh E, et al. Specific roles for the PI3K and the MEK-ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: study in the 5T33MM model. Br J Cancer. 2004;90:1076–1083. doi: 10.1038/sj.bjc.6601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 16.Rao R, Logan B, Forrest K, Roszman TL, Goebel J. Lipid rafts in cytokine signaling. Cytokine Growth Factor Rev. 2004;15:103–110. doi: 10.1016/j.cytogfr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Podar K, Tai YT, Cole CE, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278:5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 18.Maggi D, Biedi C, Segat D, Barbero D, Panetta D, Cordera R. IGF-I induces caveolin 1 tyrosine phosphorylation and translocation in the lipid rafts. Biochem Biophys Res Commun. 2002;295:1085–1089. doi: 10.1016/s0006-291x(02)00809-4. [DOI] [PubMed] [Google Scholar]
- 19.Hardin J, MacLeod S, Grigorieva I, et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- 20.Li FJ, Tsuyama N, Ishikawa H, et al. A rapid translocation of CD45RO but not CD45RA to lipid rafts in IL-6-induced proliferation in myeloma. Blood. 2005;105:3295–3302. doi: 10.1182/blood-2004-10-4083. [DOI] [PubMed] [Google Scholar]
- 21.Remacle-Bonnet M, Garrouste F, Baillat G, Andre F, Marvaldi J, Pommier G. Membrane rafts segregate profrom anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily. Am J Pathol. 2005;167:761–773. doi: 10.1016/S0002-9440(10)62049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unruh TL, Li H, Mutch CM, et al. Cholesterol depletion inhibits src family kinase-dependent calcium mobilization and apoptosis induced by rituximab crosslinking. Immunology. 2005;116:223–232. doi: 10.1111/j.1365-2567.2005.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt AB, Spindeldreher S, Kropshofer H. Clustering of MHC-peptide complexes prior to their engagement in the immunological synapse: lipid raft and tetraspan microdomains. Immunol Rev. 2002;189:136–151. doi: 10.1034/j.1600-065x.2002.18912.x. [DOI] [PubMed] [Google Scholar]
- 24.Kropshofer H, Spindeldreher S, Rohn TA, et al. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat Immunol. 2002;3:61–68. doi: 10.1038/ni750. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Qian J, Wezeman M, et al. Targeting beta(2)-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell. 2006;10:295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Biotechnology Information; GenBank. [Accessed May 2005]; http://www.ncbi.nlm.nih.gov/GenBank/
- 27.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–326. [PubMed] [Google Scholar]
- 28.Amit-Vazina M, Shishodia S, Harris D, et al. Atiprimod blocks STAT3 phosphorylation and induces apoptosis in multiple myeloma cells. Br J Cancer. 2005;93:70–80. doi: 10.1038/sj.bjc.6602637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan D, Li G, Podar K, et al. The bortezomib/proteasome inhibitor PS-341 and triterpenoid CDDO-Im induce synergistic anti-multiple myeloma (MM) activity and overcome bortezomib resistance. Blood. 2004;103:3158–3166. doi: 10.1182/blood-2003-08-2873. [DOI] [PubMed] [Google Scholar]
- 30.Jourdan M, De Vos J, Mechti N, Klein B. Regulation of Bcl-2-family proteins in myeloma cells by three myeloma survival factors: interleukin-6, interferon-alpha and insulin-like growth factor 1. Cell Death Differ. 2000;7:1244–1252. doi: 10.1038/sj.cdd.4400758. [DOI] [PMC free article] [PubMed] [Google Scholar]