Abstract
A low-oxygenic niche in bone marrow limits reactive oxygen species (ROS) production, thus providing long-term protection for hematopoietic stem cells (HSCs) from ROS stress. Although many approaches have been used to enrich HSCs, none has been designed to isolate primitive HSCs located within the low-oxygenic niche due to difficulties of direct physical access. Here we show that an early HSC population that might reside in the niche can be functionally isolated by taking advantage of the relative intracellular ROS activity. Many attributes of primitive HSCs in the low-oxygenic osteoblastic niche, such as quiescence, and calcium receptor, N-cadherin, Notch1, and p21 are higher in the ROSlow population. Intriguingly, the ROSlow population has a higher self-renewal potential. In contrast, significant HSC exhaustion in the ROShigh population was observed following serial transplantation, and expression of activated p38 mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) was higher in this population. Importantly, treatment with an antioxidant, a p38 inhibitor, or rapamycin was able to restore HSC function in the ROShigh population. Thus, more potent HSCs associated with the low-oxygenic niche can be isolated by selecting for the low level of ROS expression. The ROS-related signaling pathways together with specific characteristics of niche HSCs may serve as targets for beneficial therapies.
Introduction
Stem cells have a unique mechanism to cope with the cumulative reactive oxygen species (ROS) load, which involves increased antioxidant defenses and unique redox-dependent effects on growth and differentiation.1–5 Hematopoietic stem cells (HSCs) and the supporting cells of the stem-cell niche are predominantly located in a low-oxygen milieu of the bone marrow, which allows long-term protection from ROS-related oxidative stress.4,6,7 In the osteoblastic niche, the lowest end of an oxygen gradient within the bone marrow,6,8–10 HSCs remain quiescent and in contact with osteoblasts,11 whereas in the relatively more oxygenic vascular niche, due to the proximity to blood circulation, stem cells actively proliferate and differentiate,12–15 which might increase the intracellular ROS level.1 In the osteoblastic niche, the calcium-sensing receptor (CaR) plays a critical role in localizing HSCs to the endosteal surface, although it does not affect HSC homing,16 and osteoblast-derived factors have been suggested to improve survival of umbilical cord–derived HSCs under hypoxic conditions.10
HSCs have been enriched using a variety of techniques, including cell-surface antigen selection,17 elutriation,18 pharmacologic manipulation,19 and supravital dye,20 as well as intracellular enzyme content.21 However, none of these methods was designed to isolate quiescent HSCs located within the osteoblastic niche, which is generally considered to house the most primitive HSCs.6,8–11,14,22,23 Since it is difficult to experimentally access viable cells within the osteoblastic niche, these HSCs have not been directly or indirectly isolated by taking advantage of any of the specific properties of the niche microenvironment, such as ROS activity. Recent studies showed exhaustion of HSCs in Atm- or FoxO-deficient mice.24,25 Interestingly, HSCs derived from these mice had increased ROS levels.25,26 Thus, we hypothesized that the level of intracellular ROS may increase with proliferation or differentiation of hematopoietic cells during the very early steps of commitment (ie, activation and mobilization of cells within the long-term engrafting HSC [LT-HSC] subsets in the niche). To test this hypothesis, we took advantage of differences in the intracellular ROS level to enrich ROS low- and high-HSC populations. Our results show that the ROSlow population has a higher self-renewal activity than the ROShigh population both in vitro and in vivo. Importantly, a significant exhaustion of HSCs in the ROShigh subset was observed following the third serial transplantation, the hallmark assay for stem-cell self-renewal. The differentiation pattern in mice that received transplants of ROShigh cells was skewed in favor of myeloid differentiation comparable to that seen in aged mice. Treatment with an antioxidant, a p38 inhibitor, or a mammalian target of rapamycin (mTOR) inhibitor was able to restore functional activity of HSCs in the ROShigh population, which expressed high levels of activated p38 mitogen-activated protein kinase (MAPK) and mTOR. The ROShigh population also showed a decreased ability to adhere to osteoblast-produced matrix components compared with the ROSlow subset. The ROSlow population showed many characteristics of HSCs located within the osteoblastic niche, including quiescence and high expressions of the CaR, N-cadherin, Notch1, Bcrp, Telomerase (TERT), and p21. Our data show that the more primitive and potent, low-oxygenic niche–derived HSCs can be indirectly isolated based upon their low level of intracellular ROS expression, and these studies provide potential mechanism(s) for early steps of activation, differentiation, and eventual exhaustion of the HSCs residing outside the osteoblastic niche (ie, the ROShigh population).
Materials and methods
Mice and bone marrow cell isolation
Male C57Bl6/NCR (Ly 5.2-CD45.1) mice and female congenic mice for the CD45 locus (CD45.2) were used. Bone marrow cells were obtained by flushing tibias and femurs with α-MEM medium using a 25-G needle. The red blood cell (RBC)–lysed marrow cells were then depleted of lineage-positive cells 4 times at 4°C with rat anti-mouse monoclonal antibodies against markers CD11b (macrophage/monocyte), Ly-6G and Ly-6C (granulocyte), CD45R (B lymphocyte), CD5 or CD3e (T lymphocyte), TER-119 (erythroid cells), and 7-4 (neutrophils), and selected for CD45+ cells using magnetic sorting (Stem Cell Technologies, Vancouver, BC). We loaded samples with 5 μM DCF-DA (2′-7′-dichlorofluorescene diacetate; Molecular Probes, Carlsbad, CA) and incubated them at 37°C for 15 minutes. Before fluorescence-activated cell sorter (FACS) sorting, the cells were stained with Annexin V (AnV) to select for viable cells. AnV+ cells were usually 15% to 29% of Lin−CD45+ cells. CD45 antibodies were purchased from Pharmingen (San Diego, CA). For some experiments, we administered NAC (N-acetyl-L-cysteine) orally (1 mg/mL in drinking water). Animal experiments were approved by the Johns Hopkins University animal care committee.
Colony-forming assay and LTC-IC assay
We performed methylcellulose colony-forming assays with methylcellulose-containing medium M3434 (Stem Cell Technologies). For long-term cultures (LTCs), we cocultured 100 to 1000 cells with 3 × 104 stromal cells in Myelocult 5300 (Stem Cell Technologies) and 1 μM hydrocortisone. After 6 weeks of culture, we quantified LTC–initiating cell (LTC-IC) frequency.24 For some experiments, 100 μM NAC (Sigma Chemical, St Louis, MO), 10 μM p38 MAPK inhibitor (SB203580), 10 μM Jun N-terminal kinase (JNK) inhibitor (SP600125), 10 μM MAPK–extracellular signal–regulated kinase (MEK) inhibitor (U0126) from Biosource (Camarillo, CA) and 1 μM rapamycin (Calbiochem, San Diego, CA) were added for 2 days in the LTC.
Competitive reconstitution assay
We transplanted male CD45.1 cells into lethally irradiated (1050 cGy from a γ cell small-animal irradiator; Atomic Energy, Kanata, ON) female C57Bl6/NCR (Ly 5.1) CD45.2 congenic mice, in competition with 2 × 105 bone marrow mononuclear cells from female CD45.2 mice. A total of 2 independent experiments were performed with 10 to 2500 cells, and 10 mice for each dose were used as recipients. We monitored reconstitution of donor myeloid and lymphoid cells by staining blood cells with antibodies against CD45.1, CD45.2, CD3e, B220, Mac-1, and Gr-1.
Serial transplantation analysis
We collected CD45.1+ male donor-derived cells from 3 recipient CD45.2+ female mice 16 weeks after the first bone marrow transplantation (BMT) and pooled them. A total of 2 × 105 CD45.1 cells from primary recipient marrow were cotransplanted with 2 × 105 CD45.2 marrow cells to lethally irradiated secondary recipient CD45.2 female mice. At 16 weeks after transplantation, 2 × 105 CD45.1 cells from secondary recipient marrow were cotransplanted with 2 × 105 CD45.2 cells to lethally irradiated tertiary recipient CD45.2 female mice. A total of 5 to 10 mice from each group were used as recipients.
Flow cytometric analysis
For flow cytometric analyses, we used monoclonal antibodies specific for the following: c-Kit (2B8), Sca-1 (E13-161.7 or D7), B220 (RA3-6B2), CD3e (145-2C11), TER-119, Gr-1 (RB6-8C5), CD34 (RAM34), Mac-1 (M1/70), phosphorylated p38 (BD Biosciences, San Jose, CA) and mTOR and Bcrp (Abcam, Cambridge, MA). The cells were incubated with either rat- or mouse-derived anti-CD16/32 (BD Biosciences, San Jose, CA; and Caltag, Carlsbad, CA) before primary antibody incubation. Antibody for CaR was from Chemicon international (Temecula, CA), N-cadherin was from Cell Signaling Technology (Danvers, MA), and TERT was from Calbiochem. For cytoplasmic and nucleus antigens, 2% PFA fixed cells were permeabilized with either 0.2% Tween 20 for 15 minutes in 37°C or 90% methanol on ice and incubated sequentially for 30 minutes to overnight with primary antibodies. The cells were washed twice and stained with secondary antibodies for 30 minutes before measuring fluorescence using a FACSCalibur (BD Biosciences).
Western blot
After the FACS sorting, total proteins from different cell populations were obtained using PARIS Protein and RNA isolation kits (Ambion, Austin, TX) according to the manufacturer's protocol. Aliquots of protein extracts were loaded onto 4% to 20% Criterion Precast Gel (Bio-Rad, Hercules, CA). After electrophoresis and transfer onto Hybond-P membrane (Amersham Biosciences, Amersham, United Kingdom), Notch1, p53, and p16 were blotted, respectively, followed by β-actin to confirm equal protein loading. The antibodies used were a 1:200 dilution of rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) and a 1:5000 dilution of actin (Promega, Madison, WI). The blots were visualized using ECL Western Blotting Detection Reagents (Amersham Biosciences).
Cell-cycle analysis
To analyze the cell-cycle status by Pyronin Y (PY; Sigma) staining, cells were first stained with 7-AAD (BD, San Diego, CA) at room temperature (RT). After 20 minutes, 1 μg/mL PY was added, and cells were incubated on ice for 10 minutes. Then the cells were analyzed using FACSCalibur.
Quantitative PCR
Total RNAs were isolated using RNeasy (Qiagen, Hilden, Germany), followed by DNA-free (Ambion) treatment to get rid of residual DNA contamination. A total of 0.5 μg RNA was used for first-strand cDNA synthesis using Superscript First Strand Synthesis system (Invitrogen, Carlsbad, CA). Taqman Gene Expression Assays, Assay Mm00432448_m1 for p21, and Assay Mm00607939_s1 for β-actin control were purchased from Applied Biosystems (Foster City, CA). Platinum Taq polymerase were purchased from Invitrogen; all other reagents were from Applied Biosystems. Quadruplicates of the reaction were performed in a GeneAmp 7700 sequence detection system (Applied Biosystems) using the following program: 1 cycle at 95°C for 3 minutes and 45 cycles at 95°C for 15 seconds and 60°C for 60 seconds. The Ct values were exported into Microsoft Excel program (Redmond, WA) for further analysis.
Cell adhesion assay
Isolated cells were plated on plates coated with fibronectin, laminin, collagen IV or collagen I (BD Biosciences, Bedford, MA). Cells were allowed to adhere for 3 hours at 37°C under 5% CO2 in a humidified atmosphere.16,27 Each well was washed 5 times with PBS and fixed with methanol for 5 minutes. Cells adhered to the matrices were quantitified microscopically.
Statistical analysis
Results are expressed as means plus or minus standard error of the mean (SEM). Data were analyzed with the unpaired Student t test. A P value less than .05 was considered significant.
Results
Isolation, self-renewal, and differentiation of the ROSlow and ROShigh subsets
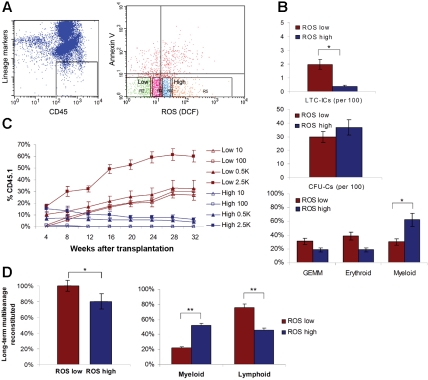
We selected ROS low and high populations from Lin−CD45+AnV− marrow cells by staining with DCF-DA as an intracellular ROS indicator. DCF-DA is a unique probe to identify viable cell ROS activity: it permeates cell membranes, is deacetylated by intracellular esterase, and becomes fluorescent upon oxidation. We collected a CD45+ population that had been lineage depleted 4 times by magnetic beads, which resulted in a 2% recovery of marrow cells (Figure 1A). Then, AnV staining was performed to remove apoptotic cells, and we selected ROSlow (< 0.2% of whole marrow) and ROShigh populations (< 0.2% of whole marrow) by FACS sorting from the CD45+Lin−AnV− cells (Figure 1A). These percentages of whole marrow are comparable to the percentage of long-term (LT)–HSCs isolated by other methods.17–21 However, it must be pointed out that we were attempting to use differential ROS activity to characterize and isolate HSCs, and not the other way around.
Figure 1.
Isolation, self-renewal, and differentiation of the ROSlow and ROShigh subsets. (A) Isolation of ROSlow and ROShigh populations. ROSlow or ROShigh Lin−CD45+AnV− marrow cells were isolated using DCF-DA cell-permeable intracellular ROS indicator. R2 indicates ROSlow; R5, ROShigh. (B) LTC-ICs at 6 weeks (top), CFU-Cs at 10 days (middle and bottom). (C) Competitive repopulation assay with 10 to 2500 cells. A total of 10 mice for each cell dose were used for the transplantation in 2 independent experiments. (D) Multilineage reconstitution potential (left). Myeloid differentiation skewing of the ROShigh population (right). Values represent the means plus or minus SEM. *P < .05; **P < .01.
To investigate whether the level of intracellular ROS correlated with stem-cell capabilities such as the self-renewal and differentiation in vitro, these populations were cultured for LTC-IC assays and colony-forming cell assays. The ROSlow population showed higher self-renewing activity and contained more LTC-ICs than the ROShigh population (Figure 1B; Table 1). However, the progenitor frequency in the ROSlow population was similar to that of ROShigh cells (Figure 1B). The ROSlow population generated more erythroid colonies and colony-forming unit–granuloycte-erythrocyte-monocyte-megakaryocte (CFU-GEMM), whereas the ROShigh population gave rise to more myeloid than erythroid colonies (Figure 1B).
Table 1.
The LTC-IC activity in the ROSlow, ROSmid, and ROShigh populations
| LTC-ICs per 100 cells | |
|---|---|
| ROSlow (R2) | 2.0 ± 0.4 |
| ROSmid-low (R3) | 1.0 ± 0.7 |
| ROSmid-high (R4) | 0.8 ± 0.5 |
| ROShigh (R5) | 0.4 ± 0.2 |
Values represent the means plus or minus the SEM. R2, etc, are as in Figure 1.
To evaluate the long-term engraftment potentials of cells in the ROSlow and ROShigh populations, we performed a competitive repopulation assay (Figure 1C). We transplanted CD45.1 male cells into lethally irradiated CD45.2 female recipients (10 mice at each cell dose) and analyzed peripheral blood at indicated time points for donor cell engraftment by costaining with CD45.1 and CD45.2. All the ROSlow cell groups showed continuously increasing percentages of donor cell engraftment up to 32 weeks after transplantation and a sustained level of engraftment from 16 weeks onward. In contrast, the ROShigh cell groups showed similar engraftment to the ROSlow cell groups after transplantation for the first 8 weeks, but eventually the level of engraftment gradually decreased (Figure 1C). All of the mice that received transplants of ROSlow cells showed multilineage engraftment (ie myeloid, B-, and T-cell differentiation) at every time point (Figure 1D). In contrast, 20% of mice that received transplants of ROShigh cells did not show T-cell lineage engraftment (Figure 1D), suggesting that this population contains cells that are less multipotent. By differential analysis, we also found a myeloid-biased differentiation for those mice that received transplants of ROShigh populations that did demonstrate multilineage long-term engraftment: At 32 weeks after transplantation, the ROSlow cell groups showed about 76% lymphocytes (B cell, 65%; T cell, 11%) and 22% myeloid cells, a similar pattern to that of normal mice that did not undergo transplantation, whereas the group that received ROShigh cells showed 46% lymphoid (B cell, 40%; T cell 6%) and 52% myeloid cell differentiation in the peripheral blood (Figure 1D). This altered repopulating activity and shift in differentiation pattern in the group that received ROShigh cells is comparable to the phenomena seen in aged mice that have been reported to contain decreased per-cell repopulating activity and myeloid skewing of differentiation.28
Association of the ROSlow subsets with properties of the osteoblastic niche–derived HSCs
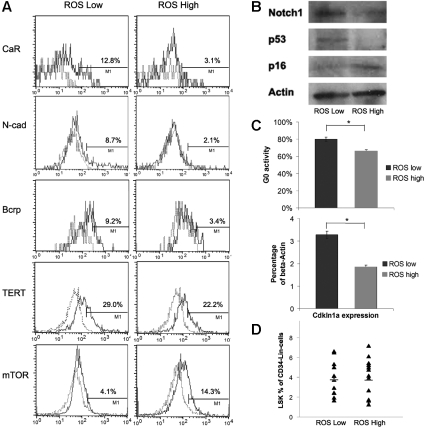
We compared the ROSlow and ROShigh subsets for several known characteristics of HSCs associated with the osteoblastic niche. The first, CaR expression, which is important for HSC localization to the endosteal niche,16 was indeed elevated in ROSlow cells compared with ROShigh cells (Figure 2A). Calcium ions are required for N-cadherin, which has been shown to be expressed in an HSC population within the osteoblastic niche,11 to form an adherent junction between HSCs and the niche.15 The expression of N-cadherin was higher in the ROSlow population than the ROShigh population (Figure 2A). Also, the expression of an ATP-binding cassette transporter, Bcrp, known to protect stem cells from exogenous and endogenous toxins and to have elevated expression within low-oxygen environments,29 was higher in the ROSlow cells. TERT activity, but not the length of telomeres, has been reported to be associated with the self-renewal potential of HSCs in mice.30 We observed that TERT expression was higher in the ROSlow population than the ROShigh population (Figure 2A). Low oxygen has been reported to inhibit mTOR and its downstream targets, and prevent cell senescence.31 mTOR is a major nutrient sensor that regulates the proliferative signaling pathways, and the activation of mTOR has been implicated in HSC division and proliferation via PTEN loss–induced AKT activation.32–34 mTOR expression was detected at a greater frequency in the ROShigh population than the ROSlow population (Figure 2A), indicating that the ROShigh population may contain more proliferating HSCs compared with the ROSlow population.
Figure 2.
Association of the ROSlow subsets with properties defining osteoblastic niche–derived HSCs. (A) Flow cytometry histograms for CaR, N-cadherin, Bcrp, TERT, and mTOR. The dotted gray lines are isotype controls. (B) Western analysis for Notch1, p53, and p16. (C) G0 activity and p21 quantitative PCR. Values represent the means plus or minus SEM. *P < .05. (D) Stem cell surface marker analysis. The variability of CD34−LSK cell frequency in 14 independent experiments. The lines represent the means.
The level of Notch1, which maintains the quiescence of HSCs through interaction with Notch ligand in the osteoblastic niche, was higher in the ROSlow population compared with the ROShigh population (Figure 2B). Severe or extended stress induces activation of p53 and leads to expression of pro-oxidant, elevated levels of ROS, and cell death.35 However, a recent report showed that a low level of p53 had an antioxidant effect in normal tissues in the absence of stress, and that p53 decreased intracellular ROS to protect the genome from oxidative damage.35 In the present study, p53 protein expression was detected at a low level in the ROSlow population, whereas it was not detected at all in the ROShigh cells (Figure 2B). This may suggest that p53, acting as an antioxidant in the ROSlow cells, contributes to the low level of ROS activity in this population. p16Ink4a has been reported as a biomarker of aging36 and is associated with HSC-repopulating defects in aged mice.28 We also observed that p16Ink4a was slightly up-regulated in the ROShigh population (Figure 2B).
Elevated ROS has been shown to block HSC quiescence.26 Therefore, we measured the cell-cycle activity of the ROSlow and ROShigh populations (Figure 2C). The proportion of cells in G0 phase in the ROShigh population, as evaluated by PY staining, was also markedly lower than that seen in ROSlow cells, supporting the idea that the ROSlow population contained more quiescent LT-HSCs and the ROShigh population contained more activated LT-HSCs. We further confirmed the quiescence of the ROSlow population using quantitative polymerase chain reaction (PCR) to detect the expression of p21, which has been known to preferentially express in slow-cycling HSCs37 (Figure 2C). Decreased expression of Cdkn1a, which encodes the cell-cycle inhibitor p21, is associated with loss of HSC function.38 The ROSlow population showed a slightly higher p21 expression consistently (Figure 2C). A certain level of p21 mRNA in the ROShigh population might be explained by high ROS-induced p21, which is related to cell senescence, or the presence of some nonactivated LT-HSC subsets within the ROShigh population. Taken together, the higher Notch1 expression (Figure 2B), cell-cycle analysis, and higher p21 mRNA expression all support the hypothesis that the ROSlow cell population is characterized as a population enriched for slow-cycling LT-HSCs (Figure 2C).
HSCs have been enriched by cell-surface marker expression patterns, and CD34−Lin−Sca+cKit+ (CD34− LSK) cells have been characterized as LT-HSCs. To determine whether the ROSlow population can also be enriched by these surface markers, we examined the cell-surface phenotype of the ROSlow and ROShigh populations (Figure 2D). Unexpectedly, the ROSlow and ROShigh subsets contained virtually equal numbers of cell-surface marker–defined LT-HSCs. Therefore, the ROSlow population might represent an overlapping yet distinct subset of LT-HSCs compared with the CD34− LSK cells. This may also suggest that LT-HSCs isolated by standard protocols represent both osteoblastic niche–located LT-HSCs and activated/divided LT-HSC subsets in other locations.
Exhaustion of HSCs during serial transplantation of the ROShigh population and the decreased adherence to the specific niche components
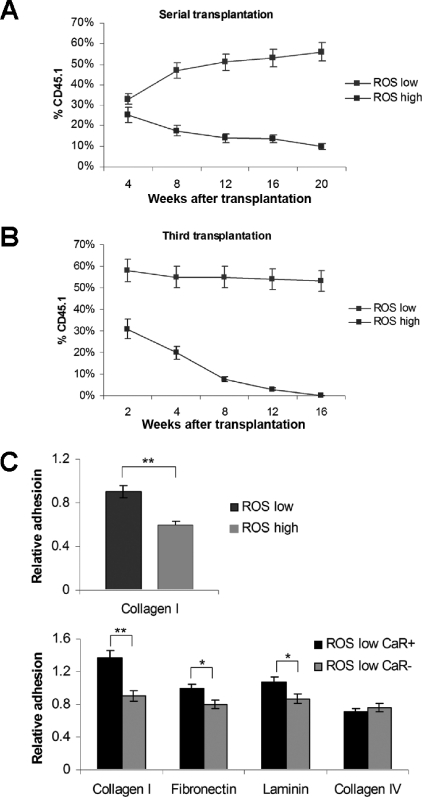
To compare the in vivo self-renewal activity of ROSlow and ROShigh populations for the presence of a more primitive LT-HSC, we performed sucessive serial transplantation studies with donor-derived cells from primary reciepients of ROSlow or ROShigh cells at 16-week intervals after transplantation. CD45.1+ donor-derived cells from the primary recipients' marrow were cotransplanted with competitor cells into lethally irradiated secondary recipients. We observed that the ROShigh population derived cells had a reduced capacity to serially rescue transplant recipients when compared with those of the ROSlow population (Figure 3A). The difference reached a highly significant level after the third transplantation, and there were no CD45.1+ donor cells detectable at 16 weeks after the third transfer in mice that had received ROShigh population–derived cell transplants (Figure 3B). This result is likely to be associated with the exhaustion of the activated LT-HSCs within the ROShigh population during these successive transplantations. The pattern is similar to the declined serial-repopulating capacity of HSCs in aged animals.39 This is also consistant with the data in Figure 1, suggesting a gradual graft loss in the ROShigh population.
Figure 3.
Exhaustion of HSCs in serial transplantation of the ROShigh population and the decreased adherence to the specific niche components. Reconstitution capacity in secondary (A) and tertiary (B) transplantation. A total of 5 to 10 mice for each condition were used as recipients. (C) Cell adhesion assay. Values represent the means plus or minus SEM and are significant at *P < .05 and **P < .01.
To assess whether this defect in long-term engraftment and self-renewal potential of the ROShigh population may be caused by a lack of CaR-dependent adherence of those cells to the osteoblastic niche, we performed cell-adhesion assays.16,27 We found a decreased ability of the ROShigh population to adhere to an extracellular matrix (ECM) component, collagen type I, which is most plentiful in bone produced by osteoblasts16 (Figure 3C). Within the ROSlow population, CaR+ cells compared with CaR− cells showed higher adhesion for the ECM molecules, collagen I, and laminin, as well as fibronectin, which is known to promote osteoblast proliferation and to bind to osteoblasts more strongly than other ECM proteins.40 However, CaR− cells did not show this defect in adhesion to an ubiquitous ECM molecule, collagen IV. Therefore, the CaR is likely to be partly responsible specifically for proper adhesion to the osteoblastic niche and the self-renewal function in the ROSlow population.
Mechanisms of HSC activation and exhaustion
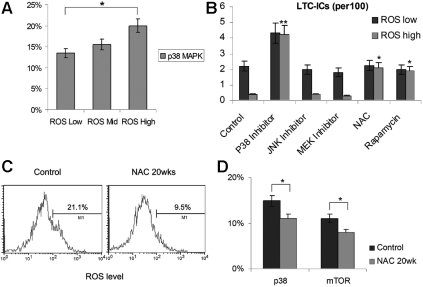
It has been recently reported that the activation of p38 MAPK, in response to elevated ROS, resulted in defects in HSC self-renewal function.26 Phosphorylated p38 assays revealed the ROShigh population contained a significantly higher number of activated p38+ cells (Figure 4A). This suggests that ROS-p38 MAPK activation might contribute to the self-renewal dysfunction we observed in the ROShigh population in vivo and in vitro. Therefore, we hypothesized that treatment with antioxidant, pharmacologic inhibition of the p38 or mTOR pathways, which was shown to be activated in the ROShigh population (Figure 2A), should restore the HSC self-renewal activity. Indeed, the antioxidant NAC, the p38 inhibitor SB203580, or the mTOR inhibitor rapamycin each restored LTC-IC activity in the ROShigh population (Figure 4B), suggesting that activating p38 MAPK and mTOR contributes to stem-cell dysfunction in the ROShigh population. In comparison, the JNK inhibitor SP600125, or the MEK (upstream of extracellular signal–regulated kinase [ERK]) inhibitor U0126 did not restore the LTC-IC activity in the ROS high population (Figure 4B). Levels of intracellular ROS in marrow cells from mice treated with NAC for 20 weeks were lower than those seen in control mice. Treatment with NAC decreased the ROShigh population (in the range of 20%-60%) and decreased p38 and mTOR activity, which were activated in the ROShigh cells (Figures 2A, 4A), compared with control mice (Figure 4C,D). However, this was not observed with shorter-term treatment (not observed at 8 weeks and not significant due to high variability at 12-16 weeks; data not shown), suggesting that high ROS levels and related signal pathways can be partially decreased only by long-term antioxidant treatment in vivo.
Figure 4.
Mechanisms of HSC activation and exhaustion. (A) Phosphorylated p38 MAPK activity; (B) LTC-IC activity with 100 μM NAC, 10 μM p38 inhibitor, 10 μM JNK inhibitor, 10 μM MEK inhibitor, and 1 μM rapamycin; ROS level (C); and phosphorylated p38 and mTOR levels (D) of viable Lin−CD45+ marrow cells after in vivo antioxidant treatment. Values represent the means plus or minus SEM. *P < .05; **P < .01.
Discussion
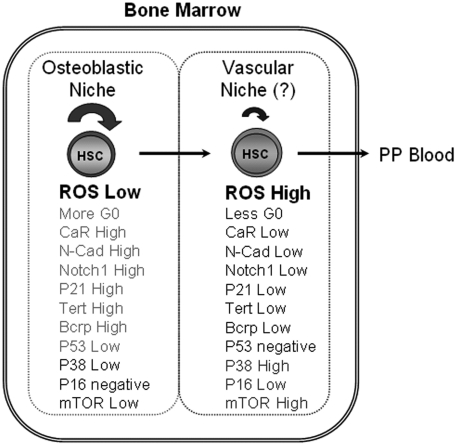
Our study revealed that it is possible to functionally enrich for primitive HSCs which reside within the low-oxygenic, osteoblastic niche using the differential activity of intracellular ROS. The ROSlow population is characterized as less-activated long-term self-renewing HSCs that express CaR, N-cadherin, Notch1, p21, p53, Bcrp and TERT, and exhibit a higher G0 activity (Figure 5). These properties support the idea that the ROSlow population might be more enriched for the quiescent HSCs physically located in the osteoblastic niche. It is important to note that this diagram (Figure 5) may not precisely reflect the relationship between the ROShigh HSC subsets and the vascular niche due to the current lack of knowledge of vascular niche specific markers relative to the accumulated evidence of markers for the osteoblastic niche.4,10,11,14–16,22,23,41–45 We found that the ROShigh subset, which may be differentially located in the more oxygenic vascular niche relative to the osteoblastic niche,4,7,14 expressed higher mTOR and activated p38 MAPK (Figures 2A,4A). These results may provide the opportunity to search for vascular niche HSC-related markers in the future. However, whether the ROS level is mainly determined by niche location (ie, oxygen gradient) or cell-intrinsic activation status remains a question to be further investigated. When comparing the properties of HSCs in the osteoblastic niche and the vascular niche (Figure 5), it is possible that the HSC status in the vascular niche represents at least an activation event, which may be responsible for the exhaustion of activated LT-HSCs during self-renewal and proliferation (Figure 3A,B) concomitant with increased and altered differentiation events (Figure 1D). We therefore propose that the metabolic activation state of HSCs initiated by moving to a more oxygenic microenvironment within the niche affects the physiologic capacity of these cells to self-renew and differentiate. Since the abnormal differentiation pattern (Figure 1D) and decreased serial-repopulating capacity (Figure 3B) in the ROShigh population showed a similarity to those in aged animals,28,39 we examined aged mice marrow for the intracellular ROS activity. The hematopoietic cells from aged mice showed approximately a 25% decrease in the ROSlow population compared with young mice, although there was only a slight increase (approximately 5%) of the ROShigh population from the aged mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Therefore, in the aged mice, the decreased ROSlow population rather than an increased ROShigh population could be responsible for the declined repopulating activity and myeloid differentiation skewing.
Figure 5.
A schematic summary of LT-HSC activation in the niche. LT-HSC distribution and the characteristics in adult bone marrow niche of normal mice. PP blood indicates peripheral blood.
It could be possible that the ROS level changes from ROSlow to ROShigh or vice versa immediately upon harvesting the marrow cells when they are suddenly exposed to higher oxygen; however, we observed that the level of ROS was stable during the isolation time period in both populations regardless of the presence of verapamil. Both ROSlow and ROShigh populations increased their ROS levels 6 weeks after culture; however, the level of ROS in the ROSlow population remained at least 20% lower than that seen in the ROShigh population (data not shown). Our study used the difference in intracellular ROS levels as a tool for isolating primitive HSCs within the niche. It was not our intention to study the effect of hypoxia on stem-cell function because pathologic hypoxia or ischemia cannot be directly compared with a physiologically low-oxygenic environment in the normal tissue (ie, marrow). Importantly, not only the low-oxygen condition of the osteoblastic niche but also the niche-specific HSC characteristics (ie, CaR, N-cadherin, Bcrp, p21, and Notch1) were studied to functionally enrich and isolate such quiescent niche HSCs. Considering successive serial transplantations, during which these cells were experiencing a low-oxygen environment in vivo and reoxygenation ex vivo several times, the low-oxygenic milieu would not be the only factor contributing to maintain HSC self-renewal (Figure 3B). This is supported by differences in protein and gene analysis that are specific for the HSCs in the osteoblastic niche (Figure 2) and the cell adhesion to the osteoblastic niche components (Figure 3C). Therefore, the difference in stem-cell function within the 2 populations we observed remain even after the ex vivo oxygen exposure during the experiments.
One of the cyclin-dependent kinase inhibitors, p16Ink4a, is expressed at higher levels in the ROShigh cells than in the ROSlow cells (Figure 2B). However, levels of cyclin-dependent kinase inhibitor 1a (p21) and tumor suppressor p53 were higher in the ROSlow cells (Figure 2B,C). In the ROSlow population enriched for long-term self-renewing cells, the role of p21 seems to maintain the quiescence,37 and the low level of p53 may have more to do with antioxidant function and decreasing the intracellular ROS level.35 Since hematopoietic exhaustion has been reported to be accelerated in the absence of p21 in irradiated hosts,46 the lower level of p21 in the ROShigh population (Figure 2C) might affect the exhaustion of HSCs in serial transplant recipients (Figure 3A,B). In the ROShigh population, p16Ink4a might act as the ROS regulatory protein, as well as decrease the repopulating activity of HSCs,28 rather than act as a cell-cycle inhibitor. It is also possible that the cell-cycle parameters of each population are determined by many other proteins in addition to p21 and p16Ink4a. Our results support the different roles played by cell cycle–related proteins in different cell types, even within multiple HSCs of varying ages and potentials for proliferation and differentiation.
Notch signaling has been implicated in the maintenance of undifferentiated HSCs.47 Disruption of this pathway causes accumulation of ROS in activated T cells.48 We observed higher expression of Notch1 in the ROSlow HSCs, suggesting that Notch signaling may also play a role in maintaining this primitive HSC population. Whether Notch signaling is responsible for maintaining low ROS level in bone marrow cells has, however, not yet been investigated.
We observed that the exposure of the marrow cell populations to compounds that inhibit p38, promote antioxidant activity, or inhibit the mTOR were effective in restoring HSC function in the ROShigh population (Figures 2A,4). The results of the p38 inhibitor and antioxidant compounds are consistent with the analysis of Atm knockout mice in which the ROS-p38 pathway contributed to exhaustion of the stem-cell population.26 ROS have also been known to activate the PI3K-Akt-mTOR pathway; this is preventable by rapamycin.49 The different LTC-IC activity observed in the ROSlow and ROShigh populations is likely the result of signaling events that require both pathways mediated by p38 MAPK and mTOR, since blocking either one of them eliminates the difference (Figure 4B). In addition, blocking p38 further enhances LTC-ICs in both ROSlow and ROShigh populations, suggesting an additional function of p38 MAPK, independent of mTOR, which involves the HSC self-renewal activity. Although in vitro HSC self-renewal function was almost fully corrected with the NAC treatment (Figure 4B), the level of ROS in marrow cells and phosphorylated p38 MAPK, and mTOR activity in vivo, were only partially restored (Figure 4C,D). This may reflect the presence of an in vivo inhibitory mechanism(s) against the effects of NAC on ROS-p38 MAPK or ROS-mTOR pathways. In our study, the ROShigh population contained more mTOR and phosphorylated p38+ cells (Figure 4A), and this activation might occur only at the stem-cell level.26 If so, the restoration of HSC function by treatment with those inhibitors might also occur at the HSC level, not at the level of later hematopoietic progenitors (Figure 4B). Importantly, functional alteration of ROShigh cells by antioxidant, p38 inhibitor, or rapamycin indicates that in certain cases of bone marrow failure or cancers that are accompanied by increased oxidative stress, these drugs or cellular therapy using a ROSlow stem-cell population may have therapeutic potentials. It will be important to determine if our stem-cell selection can be adapted to the purification of human marrow and thereby establish a novel functional isolation method for human HSCs.
In summary, we provide a new approach to functionally isolate primitive HSCs that are capable of more durable long-term self-renewal. These ROSlow cells have a number of characteristics of quiescent HSCs that might be located in the osteoblastic niche, including long-term serial-repopulating activity, less cycling, and expression of CaR, N-cadherin, Notch1, p21, p53, Bcrp, and TERT. Remarkably, a p38 inhibitor, rapamycin, and antioxidant compounds can rescue the ROS-induced stem-cell dysfunction (ie, loss of self-renewal) in the ROShigh population, which showed higher expressions of p38 and mTOR and decreased adherence to the osteoblastic niche matrix components. This suggests that intracellular ROS levels and the related signal transduction pathways, together with specific characteristics that define the osteoblastic niche, are responsible for long-term self-renewal as well as activation, proliferation, and differentiation of HSCs. Some components of these pathways and the functionally selected niche HSC subsets may serve as potential targets for beneficial therapies for human diseases, especially many hematologic malignancies.
Supplementary Material
Acknowledgments
We thank Drs. Stephen Baylin, Neil Watkins, Charlie Rudin, David Berman, and Christine Hann for helpful discussions and critical evaluation of the manuscript. We thank Michael Collector and Bill Schuler for technical assistance.
This work was supported by the Institute for Cellular Engineering at Johns Hopkins and grants R01HL54330 and RO1DK070971 from the National Institutes of Health.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.-Y.J. designed and carried out experiments and wrote paper. S.J.S. helped with planning and preparing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoon-Young Jang or Saul J. Sharkis, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine, 1550 Orleans St, Baltimore, MD; e-mail: yjang3@jhmi.edu or ssharkis@jhmi.edu.
References
- 1.Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22:962–971. doi: 10.1634/stemcells.22-6-962. [DOI] [PubMed] [Google Scholar]
- 2.Csete M, Walikonis J, Slawny N, et al. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 3.Limoli CL, Rola R, Giedzinski E, Mantha S, Huang TT, Fike JR. Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci U S A. 2004;101:16052–16057. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Piccoli C, Ria R, Scrima R, et al. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. J Biol Chem. 2005;280:26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 6.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 8.Deldar A, Lewis H, Weiss L. Bone lining cells and hematopoiesis: an electron microscopic study of canine bone marrow. Anat Rec. 1985;213:187–201. doi: 10.1002/ar.1092130211. [DOI] [PubMed] [Google Scholar]
- 9.Antoniou ES, Sund S, Homsi EN, Challenger LF, Rameshwar P. A theoretical simulation of hematopoietic stem cells during oxygen fluctuations: prediction of bone marrow responses during hemorrhagic shock. Shock. 2004;22:415–422. doi: 10.1097/01.shk.0000142185.88094.88. [DOI] [PubMed] [Google Scholar]
- 10.Dao MA, Creer MH, Nolta JA, Verfaillie CM. Biology of umbilical cord blood progenitors in bone marrow niches. Blood. 2007;110:74–81. doi: 10.1182/blood-2006-08-034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 12.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 13.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suda T, Arai F, Shimmura S. Regulation of stem cells in the niche. Cornea. 2005;24:S12–S17. doi: 10.1097/01.ico.0000178742.98716.65. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31:589–595. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 17.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 18.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 19.Szilvassy SJ, Cory S. Phenotypic and functional characterization of competitive long-term repopulating hematopoietic stem cells enriched from 5-fluorouracil-treated murine marrow. Blood. 1993;81:2310–2320. [PubMed] [Google Scholar]
- 20.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 21.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehryogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 22.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 23.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 25.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 27.Koenigsmann M, Griffin JD, DiCarlo J, Cannistra SA. Myeloid and erythroid progenitor cells from normal bone marrow adhere to collagen type I. Blood. 1992;79:657–665. [PubMed] [Google Scholar]
- 28.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;43:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 30.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cell is associated with self renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 31.Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 32.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 35.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 38.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 40.Garcia AJ, Ducheyne P, Boettiger D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J Biomed Mater Res. 1998;40:48–56. doi: 10.1002/(sici)1097-4636(199804)40:1<48::aid-jbm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 41.Yin T, Li L. The stem cell niches in bone J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;6:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Bilbe G, Roberts E, Birch M, Evans DB. PCR phenotyping of cytokines, growth factors and their receptors and bone matrix proteins in human osteoblast-like cell lines. Bone. 1996;19:437–445. doi: 10.1016/s8756-3282(96)00254-2. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Yuan Y, Shen H, Cheng T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood. 2006;107:1200–1206. doi: 10.1182/blood-2005-02-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan AW, Rattis FM, DiMascio LN, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 48.Bheeshmachar G, Purushotaman D, Sade H, Gunasekharan V, Rangarajan A, Sarin A. Evidence for a role for notch signaling in the cytokine-dependent survival of activated T cells. J Immunol. 2006;177:5041–5050. doi: 10.4049/jimmunol.177.8.5041. [DOI] [PubMed] [Google Scholar]
- 49.Tu VC, Bahl JJ, Chen QM. Signals of oxidant-induced cardiomyocyte hypertrophy: key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. J Pharmacol Exp Ther. 2002;300:1101–1110. doi: 10.1124/jpet.300.3.1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.