Abstract
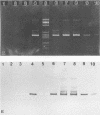
A reverse transcription-PCR method was developed to detect enterovirus (EV), hepatitis A virus (HAV), and rotavirus (RV) RNAs in shellfish and sediment. The method was first tested under experimental conditions by using virus-spiked shellfish to evaluate assay sensitivity. The use of CC41 cellulose was found to be efficient for removing inhibitors of RV detection. For sediment samples, a Sephadex column was used to allow the detection of EV and HAV RNAs. The specificity of amplified products was controlled by hybridization with digoxigenin-labeled oligoprobes. The method was then applied to naturally contaminated shellfish and sediments. EV, HAV, and RV RNAs were detected in 22, 14, and 20% of the shellfish samples, respectively. No relationship between viral contamination and bacterial contamination was found. When viral RNAs (HAV or EV) were detected in sediments, they were also detected in shellfish.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszadegan M., Huber M. S., Gerba C. P., Pepper I. L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993 May;59(5):1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar R. L., Metcalf T. G., Neill F. H., Estes M. K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N. M., Tracy S., Gauntt C. J., Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990 May;28(5):843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crance J. M., Passagot J., Biziagos E., Deloince R. Continuous production of hepatitis A virus in PLC/PRF/5 cell cultures: use of antigen for serology. J Virol Methods. 1987 Nov;18(2-3):193–203. doi: 10.1016/0166-0934(87)90124-8. [DOI] [PubMed] [Google Scholar]
- Desenclos J. C., Klontz K. C., Wilder M. H., Nainan O. V., Margolis H. S., Gunn R. A. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am J Public Health. 1991 Oct;81(10):1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Sears J., Schael I. P., White L., Garcia D., Lanata C., Kapikian A. Z. Identification of human rotavirus serotype by hybridization to polymerase chain reaction-generated probes derived from a hyperdivergent region of the gene encoding outer capsid protein VP7. J Virol. 1990 Aug;64(8):4021–4024. doi: 10.1128/jvi.64.8.4021-4024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Glass R. I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B. K., Bhan M. K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992 Jun;30(6):1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B. B., Koch W. H., Cebula T. A. Detection of hepatitis A virus in Mercenaria mercenaria by coupled reverse transcription and polymerase chain reaction. Appl Environ Microbiol. 1993 Sep;59(9):2765–2770. doi: 10.1128/aem.59.9.2765-2770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Allen J. R., Glass R. I., Fang Z. Y., Bremont M., Cohen J., McCrae M. A., Saif L. J., Sinarachatanant P., Caul E. O. Detection of group B and C rotaviruses by polymerase chain reaction. J Clin Microbiol. 1991 Mar;29(3):519–523. doi: 10.1128/jcm.29.3.519-523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J., Ticehurst J., Flehmig B. Detection of hepatitis A virus in sewage sludge by antigen capture polymerase chain reaction. Appl Environ Microbiol. 1993 Oct;59(10):3165–3170. doi: 10.1128/aem.59.10.3165-3170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M. L., Kang L. Y., Zhou T. K., Hu M. D., Pan Q. C., Fu T. Y., Huang Y. S., Hu S. L. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J Infect Dis. 1991 Nov;164(5):852–859. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Combined immunoaffinity cDNA-RNA hybridization assay for detection of hepatitis A virus in clinical specimens. J Clin Microbiol. 1985 Dec;22(6):984–989. doi: 10.1128/jcm.22.6.984-989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Dubrou S., Prevot J., Marechal J., López-Pila J. M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl Environ Microbiol. 1993 Apr;59(4):1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämmerer U., Kunkel B., Korn K. Nested PCR for specific detection and rapid identification of human picornaviruses. J Clin Microbiol. 1994 Feb;32(2):285–291. doi: 10.1128/jcm.32.2.285-291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guyader F., Apaire-Marchais V., Brillet J., Billaudel S. Use of genomic probes to detect hepatitis A virus and enterovirus RNAs in wild shellfish and relationship of viral contamination to bacterial contamination. Appl Environ Microbiol. 1993 Nov;59(11):3963–3968. doi: 10.1128/aem.59.11.3963-3968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. D., Metcalf T. G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988 Aug;54(8):1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert D. U., Stewien K. E. Detection and distribution of rotavirus in raw sewage and creeks in São Paulo, Brazil. Appl Environ Microbiol. 1993 Jan;59(1):140–143. doi: 10.1128/aem.59.1.140-143.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzo S., Bagnarelli P., Giacca M., Manzin A., Varaldo P. E., Clementi M. Absolute quantitation of viremia in human immunodeficiency virus infection by competitive reverse transcription and polymerase chain reaction. J Clin Microbiol. 1992 Jul;30(7):1752–1757. doi: 10.1128/jcm.30.7.1752-1757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard Scott L., Paul John H. Detection of Gene Expression in Genetically Engineered Microorganisms and Natural Phytoplankton Populations in the Marine Environment by mRNA Analysis. Appl Environ Microbiol. 1991 Jun;57(6):1721–1727. doi: 10.1128/aem.57.6.1721-1727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Brown V. K., Khanna B. Altered hepatitis A VP1 protein resulting from cell culture propagation of virus. Virus Res. 1989 Jul;13(3):207–212. doi: 10.1016/0168-1702(89)90016-6. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severini G. M., Mestroni L., Falaschi A., Camerini F., Giacca M. Nested polymerase chain reaction for high-sensitivity detection of enteroviral RNA in biological samples. J Clin Microbiol. 1993 May;31(5):1345–1349. doi: 10.1128/jcm.31.5.1345-1349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub T. M., Pepper I. L., Abbaszadegan M., Gerba C. P. A method to detect enteroviruses in sewage sludge-amended soil using the PCR. Appl Environ Microbiol. 1994 Mar;60(3):1014–1017. doi: 10.1128/aem.60.3.1014-1017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Sobsey M. D., Sangermano L. R., Palmer C. J. Simple method of concentrating enteroviruses and hepatitis A virus from sewage and ocean water for rapid detection by reverse transcriptase-polymerase chain reaction. Appl Environ Microbiol. 1993 Oct;59(10):3488–3491. doi: 10.1128/aem.59.10.3488-3491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Eiden J., Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990 Jun;28(6):1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Harbour D., McCrae M. A. The application of polymerase chain reaction to the detection of rotaviruses in faeces. J Virol Methods. 1990 Jan;27(1):29–37. doi: 10.1016/0166-0934(90)90143-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y. J., Estes M. K., Jiang X., Metcalf T. G. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991 Oct;57(10):2963–2968. doi: 10.1128/aem.57.10.2963-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]