Abstract
This paper examined the association between grip type, hand use, and fingerprint patterns in a sample of captive chimpanzees. Grip type for simple reaching was assessed for the left and right hand and classified as thumb-index, middle-index, or single-digit responses. Fingerprint patterns were characterized as whorls, loops, or arches on each finger. The results indicated that chimpanzees exhibit significantly more thumb-index responses for the right compared to the left hand. In addition, thumb-index responses were more prevalent for subjects that had a whorl compared to a loop or arch on their thumb. The results suggest that fingerprint patterns are associated with individual differences in grasping type in chimpanzees as well as some variation in hand use.
Keywords: handedness, chimpanzees, dermatoglyphics, laterality
Comparative assessment of paw and hand preferences in animals and humans remains a topic of continued research (Bradshaw and Rogers, 1993; Marchant and McGrew, 1991; Ward and Hopkins, 1993). Historically, it was held that population-level asymmetries in limb use are a uniquely human phenomenon (Ettlinger, 1988; Fagot and Vauclair, 1991; MacNeilage et al., 1987; Warren, 1980), but recent research in a host of animals clearly challenges this long held belief (Bradshaw and Rogers, 1993). There is now very good evidence of population-level asymmetries in perceptual, motor, and cognitive functions in a number of vertebrates (Rogers and Andrews, 2002), but what remain largely unexplored, particularly in nonhuman primates, are the factors and mechanisms that influence the expression of handedness. Of specific interest to this study is the association between hand use, dermatoglyphics, and grip type.
Recent studies in chimpanzees and other great apes showed a significant relationship between grip type and hand use for simple reaching. At least three separate studies found increased preferential use of the right hand associated with the adoption of a thumb-index finger-grasping response compared to other grasping techniques (Christel, 1994; Christel et al., 1998; Hopkins et al., 2002; Jones-Engel and Bard, 1996; Tonooka and Matsuzawa, 1995). In all of these studies, hand preference as well as grip type was characterized in individual subjects while they grasped food items. However, one limitation to these previous studies is that hand preference and grip type were not dissociated from an experimental standpoint. In particular, within a fixed number of total trials, the distribution of different kinds of grips was compared between hands rather than controlling for the number of grasping responses executed by each hand and comparing the total number of different grasping techniques. The primary limitation of the previous studies is that thumb-index responses were observed most often, but subjects also used their right hand significantly more often. In this study, three different types of grips were recorded in chimpanzees when picking up a small food item (for a much more elaborate system of grasping postures, see Marzke, 1997). The total number of responses recorded from each hand was controlled for, which allowed specific comparisons of different grip types between the left and right hands. Based on previous findings on grip type and hand use in chimpanzees (Hopkins et al., 2002; Jones-Engel and Bard, 1996; Tonooka and Matsuzawa, 1995), it was hypothesized that precision grips using the thumb and index finger would be significantly higher for the right compared to the left hand.
In addition to examining the relation between grip type and hand use, we sought to assess whether individual differences in grip type were associated with variation in dermatoglyphics, and specifically fingerprint patterns. Commonly known as fingerprints, dermatoglyphics or epidermal ridge configurations are thought to aid in grip and sensation for gross and fine motor skills, as well as to enhance protection of the epidermis against sustained use (Cummins and Midlo, 1926; Holt, 1968; Loesch, 1983).
Humans show three distinct fingerprint patterns, including whorls, arches, and loops. Two principal landmarks are used to categorize fingerprint patterns and to systematically count the ridges that form these patterns (Fig. 1). The first landmark is the pattern core, which is the central point encompassed by the innermost ridges. The second landmark is the triradius or union of three dermal ridge systems, which can occur more than once in a single pattern. The core and triradius are formed and surrounded by concentric ridges also known as radiants (Schaumann and Alter, 1976). Various positions and combinations of these landmarks produce countless patterns (see Fig. 1 for examples). These landmarks can also be used to systematically quantify the density of dermal ridges for most dermatoglyphic patterns (Schaumann and Alter, 1976).
Fig. 1.
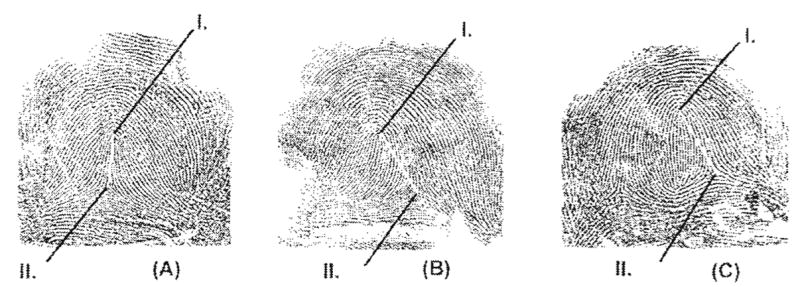
Examples of each fingerprint pattern. I, core; II, triradius: landmarks used to define each fingerprint pattern. A: Loop. B: Arch. C: Whorl.
Striking similarities in dermal ridge patterns between human and nonhuman subjects have allowed for the same landmarks and ridge count methods to be used for comparative studies. For example, parallels in pattern description such as the arch-loop-whorl system were reported for humans compared to both chimpanzees (Biegert, 1971; Cummins and Spragg, 1938) and monkeys (Newell-Morris et al., 1982). Unfortunately, most studies of dermatoglyphics in nonhuman primates have been descriptive and have not considered the functional role that variation in fingerprint patterns may have on behavior, notably grasping.
Therefore, the second purpose of this study was to evaluate whether individual differences in grip type could be explained by variation in fingerprint patterns. Studies in human subjects demonstrated that mechanoreceptors involved in grasping innervate the dermal ridges of the fingers (Johnson, 2001). A recent study in monkeys showed that there was a higher proportion of some mechanorecptors in the thumb compared to the index and fifth digit (“pinky”) (Paré et al., 2002). Although it not yet known whether there is greater innervation of mechanoreceptors in different fingerprint patterns, it was demonstrated that the number of ridges is significantly higher for whorls compared to arches and loops (Mi et al., 1982). Based on these observations, we hypothesized that variation in fingerprint patterns between fingers might have some impact on the type of grip employed by an individual. For example, in thumb-index responses, the food item is grasped by abducting the thumb against the side of the index finger (and in some cases the tip of the index fingers). In this case, the primary sensory and motor feedback is coming from the mechanoreceptors of the thumb rather than the other digits. If an individual had a whorl on the thumb, which has more ridges (and presumably more receptors), then it might be hypothesized that a thumb-index grip might be more common for these individuals compared to others with different fingerprint patterns. Therefore, the goal of this study was not only to more systematically access the relationship between grip type and hand use, but also to look at the role variation in fingerprint patterns may have on the employment of one grip type over another.
METHODS: GRIP TYPE AND HAND USE ASSESSMENT
Subjects
Subjects were captive chimpanzees (Pan troglodytes) housed at the Yerkes National Primate Research Center (YNPRC) of Emory University. For grip type assessment, data were available from 138 individuals, including 81 females and 57 males. Subject ages ranged from 5–45 years.
Procedure
Hand use and grip type were recorded for simple reaching. For each test, the experimenter would throw a handful of food items into the subject’s home cage. The hand used and type of grip employed were recorded for each response. The food items used were Honey Nut Cheerios or Fruit Loops, which measure 1 cm in diameter and are a highly preferred food item. Grip type for each reaching response was characterized as either thumb-index, middle-index, or single-digit. Any grasping response in which the subjects abducted the thumb to the index finger to grip the food was recorded as a thumb-index response. Reaching was recorded as middle-index grip when subjects grasped the food between the index and middle finger with the hand either in a prone or supine position. Thumb-index and middle-index grip types were by far the most common (accounting for over 97% of responses), but subjects occasionally engaged in what was recorded as single-digit responses. Single-digit responses were instances in which the chimpanzees used one finger to press down hard enough on the food item so that it stuck while being taken to the mouth.
Instances in which the subject used his or her mouth to pick up the food item were not recorded. Both left- and right-hand responses were recorded until 30 responses were obtained for each hand. Food items were continuously thrown into the cage until 30 responses were obtained from each hand. Note that if a subject was reluctant to use one hand over the other, the experimenter would intentionally throw the food items into the cage in a location that would encourage the subjects to use the less dominant hand. In this way, it was assured that 30 responses for each hand would be obtained from each subject. At the end of the experiment, the total number of thumb-index, middle-index, and single-digit responses were summed for both the left and right hands.
RESULTS
In this analysis, the primary interest was whether or not grip type changed as a function of hand use. A 2 × 2 × 3 mixed-model analysis of variance was performed with hand (right or left) and grip type (index-thumb, index-middle, or single-digit) as the repeated-measures and sex as the between-group variable. A significant two-way interaction was found between hand and grip type (F(2,228) = 10.49, P < 0.001). Depicted in Figure 2 is the mean number of index-thumb, middle-index, and single-digit responses for each hand. Post hoc analysis using paired t-tests revealed significantly more index-middle grips performed with the left hand (mean = 13.99) compared to the right hand (mean = 11.28) (t(115) = − 3.36, P < 0.01). Conversely, there were significantly fewer index-thumb grips performed with the left hand (mean = 15.51) compared to the right hand (mean = 18.30) (t(115) = − 3.29, P < 0.01). No significant difference was found in hand use for single-digit responses.
Fig. 2.
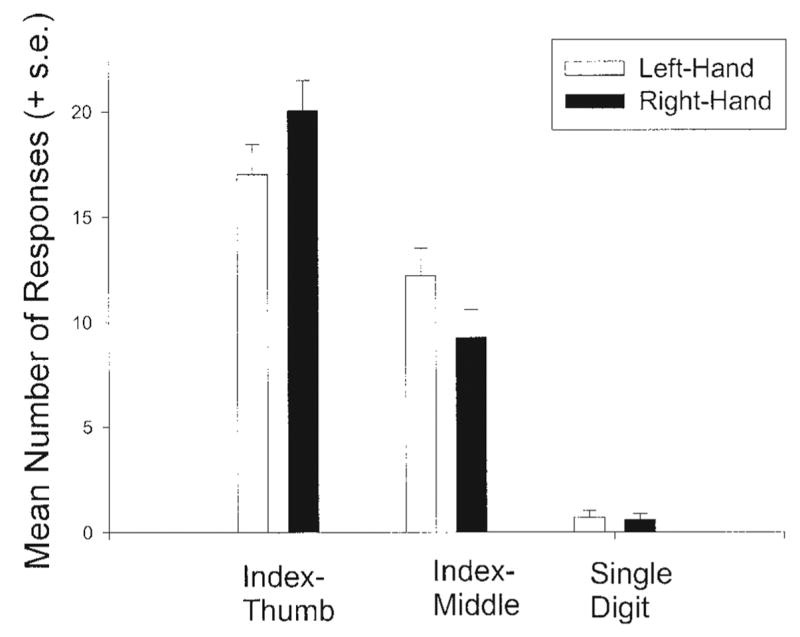
Mean number of responses for each grip type for left and right hands.
DISCUSSION
Regarding hand use and grip type, chimpanzees show a greater frequency in thumb-index grasping with the right compared to the left hand. In addition, chimpanzees show a greater frequency in index-middle finger grasping in the left compared to the right hand. These results are largely in agreement with previous findings in chimpanzees (Hopkins et al., 2002; Tonooka and Matsuzawa, 1995; but see Jones-Engel and Bard, 1995), but the present findings are the first to demonstrate a functional specialization in the use of different grip morphologies between the right and left hands, when the frequency of right and left hand use was controlled for in responding.
METHODS: GRIP TYPE AND FINGERPRINT PATTERN
Subjects
Subjects were captive chimpanzees (Pan troglodytes) housed at the Yerkes National Primate Research Center (YNPRC) of Emory University. The number of subjects in each aspect of the study varied somewhat due to the availability and quality of fingerprints obtained. Useable fingerprint data were obtained from 150 chimpanzees, including 82 females and 68 males. Our analyses were restricted to the 116 chimpanzees used in experiment 1 (see above).
Procedure
Images of fingerprints were obtained when subjects were anesthetized during routine medical surveys between June 1998–July 2000. In preparation for obtaining a print, the palm of the hand and volar surface of the fingers were cleaned using a surgical iodine scrub. Excess foam and moisture were absorbed with paper towels, and any remaining oil or residue was removed with an isopropyl alcohol pad. Once the subject’s hands were dry, a sheet of carbon paper was rubbed across the volar and lateral surfaces of the apical digit, and a piece of transparent mailing tape (approximately 2 × 4 inches) was wrapped horizontally around the terminal phalanx. Light pressure was applied to ensure contact with the entire fingertip and to prevent wrinkles in the tape. The tape was then carefully peeled back from the finger and attached to a prepared data sheet, where it was labeled from 1–5 (thumb = digit I, index finger = digit II, middle finger = digit III, ring finger = digit IV, and little finger or pinky = digit V).
Complications during the medical examination of some subjects, such as development of a grip during anesthesia (with fingers tightly clenched), or palm and fingertip sweating, caused some poor-quality print records. Deep cracks in the distal flexion crease, vertical and circular folds on the apical volar surface, and calluses and scars also made print analyses difficult for some individuals. Two subjects, one male and one female, were discarded from the data collection due to indistinct images for all fingers, resulting in an overall sample size of 150 individuals.
Shown in Table 1 are the distributions of each fingerprint type as a function of hand and finger in the chimpanzee sample. For each digit, the distributions of each fingerprint type were compared against a chance distribution, using a chi-square goodness-of-fit test. The results revealed that, with the exception of digit V on both the left and right hand, subjects predominantly showed a greater frequency of whorls compared to arches and loops. For digit V, there were roughly equal numbers of loops and whorls. Chi-square tests of independence failed to reveal any significant interactions between sex and fingerprint patterns for any of the digits on either the left or right hand. Because there were so few cases of either loops or arches for some of the fingers that it precluded a direct comparison of the three types of print patterns, for further analysis, individuals with either arches or loops on a given digit were combined into one category for comparison with individuals with a whorl (the pattern of particular interest) on a given digit.
TABLE 1.
Frequency of fingerprint patterns for each hand and digit
| Whorl | Loop | Arch | Chi-square | P | |
|---|---|---|---|---|---|
| Right hand digit | |||||
| I | 80 | 15 | 16 | 74.97 | 0.001 |
| II | 110 | 31 | 3 | 128.29 | 0.001 |
| III | 107 | 29 | 10 | 108.59 | 0.001 |
| IV | 98 | 43 | 6 | 87.47 | 0.001 |
| V | 74 | 73 | 1 | 71.04 | 0.001 |
| Left hand digit | |||||
| I | 81 | 12 | 18 | 78.97 | 0.001 |
| II | 91 | 49 | 2 | 83.76 | 0.001 |
| III | 97 | 38 | 11 | 79.49 | 0.001 |
| IV | 95 | 47 | 4 | 85.16 | 0.001 |
| V | 50 | 99 | 0 | 16.11 | 0.01 |
RESULTS
For this analysis, the frequency of thumb-index responses for each hand was compared as a function of fingerprint patterns using an analysis of variance (ANOVA). For analysis of fingerprint patterns on the right hand, the number of thumb-index responses of the right hand served as the dependent variable. Similarly, for analysis of the fingerprint patterns on the left hand, the number of thumb-index responses for the left hand served as the dependent variable. The mean number of thumb-index responses for each finger and fingerprint pattern is shown in Table 2. As can be seen, for both the right and left hands, significant effects of fingerprint pattern were found for the thumb but not for any of the remaining digits. For both the right and left hand, subjects with a whorl on their thumb made significantly more thumb-index responses than subjects with either a loop or arch on their thumb.
TABLE 2.
Mean number of thumb-index responses as a function of fingerprint pattern and digit
| Whorl | Loop or arch | F-value | P | |
|---|---|---|---|---|
| Right hand digit | ||||
| I | 20.75 | 15.07 | F(1,82) = 5.13 | 0.03 |
| II | 18.81 | 16.77 | F(1,109) = 0.12 | 0.73 |
| III | 18.62 | 17.28 | F(1,110) = 0.13 | 0.72 |
| IV | 18.54 | 17.78 | F(1,111) = 0.07 | 0.79 |
| V | 18.66 | 17.80 | F(1,110) = 0.14 | 0.71 |
| Left hand digit | ||||
| I | 18.33 | 11.83 | F(1,82) = 5.26 | 0.03 |
| II | 14.57 | 16.92 | F(1,106) = 0.81 | 0.37 |
| III | 15.62 | 14.79 | F(1,110) = 0.10 | 0.76 |
| IV | 14.94 | 16.24 | F(1,110) = 0.18 | 0.67 |
| V | 14.36 | 16.16 | F(1,111) = 0.50 | 0.48 |
DISCUSSION
The fingerprint characteristics observed in our sample are consistent with those previously reported by Cummins and Spragg (1938) in a sample of 26 chimpanzees. Although we assessed fingerprint patterns in many more chimpanzees than did Cummins and Spragg (1938), the relative proportion of subjects that possessed a whorl fingerprint pattern was comparable between samples. For example, in our sample as well as in that of Cummins and Spragg (1938), whorls were the most common fingerprint pattern, and the relative proportion of whorls was highest on the thumb compared to the remaining digits.
GENERAL DISCUSSION
There were two main findings in this study. First, chimpanzees in the current study showed significantly higher frequencies of thumb-index grasping responses for the right compared to the left hand. Second, individual differences in the use of thumb-index grasping responses were associated with the presence/absence of a whorl fingerprint pattern on the thumb but not the remaining digits. More specifically, individuals who had a whorl fingerprint on the thumb of a given hand made significantly more thumb-index responses with that hand than individuals with a loop or whorl on their thumb.
Why the interaction between hand use and grip type exists remains unclear. One interpretation is that the evidence of increased use of a thumb-index response in the right hand reflects an inherent bias in motor skill controlled by the left hemisphere in chimpanzees. Studies in human subjects showed that the right hand is better at performing skilled actions compared to the left (Annett, 1985, 1992), and our findings appear to be consistent with this observation. However, this explanation assumes that thumb-index grip type confers some advantage over other grip morphologies, and this is not so clear from the existing literature. Christel (1993, 1994) did not provide error rates for different grasping techniques in her otherwise excellent descriptive analysis of grip morphologies in various primate species. Hopkins et al. (2002) found that the error rates (with an error being defined as any failed grasping attempt in which the desired object is dropped) for thumb-index grips were significantly lower than index-middle and single-digit grip techniques, but these findings are difficult to interpret because the rate of different grasping techniques varied widely across subjects. Thus, the data are suggestive of a left-hemisphere advantage in motor skill that is manifest in different types of grasping techniques employed by chimpanzees. Further research is needed to clarify the advantages or disadvantages in motor skill associated with different grasping techniques before any definitive conclusions can be drawn.
An alternative explanation for the interaction between hand use and grip type may be that differences could exist in the morphology of the left and right hands, particularly in the ratio of the size of the index finger to the thumb, which could potentially have an effect on the type of grip employed by each hand. Napier and Napier (1967) described in great detail the opposability index for various primate species and how it relates to the evolution of the hand and hand functions associated with grasping and locomotion. The opposability index reflects the ratio in the size of the thumb relative to the index finger. Although this has not been reported in the literature, it might be that differences in the opposability index between the right and left hands account for the within-subject variation in grasping. In this scenario, differences in the physical size of either the index finger or thumb between hands would promote the use of different grasping techniques.
As far as we know, the findings in this study are the first evidence linking different fingerprint patterns with individual differences in grip type in grasping for food. In general, our findings support the idea that greater fingerprint complexity on the thumb results in higher frequencies of thumb-index responses. This explanation assumes that more complex fingerprint patterns have either a greater number or asymmetry in the distribution of mechanoreceptors involved in sensory and motor functions associated with grasping. Unfortunately, there are no published studies on receptor distributions or occurrence in the fingertips of the hands, in relation to variation in fingerprint patterns in chimpanzees, although it was reported that the distribution of Meissner’s corpuscles is comparable between chimpanzees and humans (Biegert, 1971). In monkeys, dense populations of Meissner’s corpuscles were found on the thumb, index, and little fingers (Paré et al., 2002). Moreover, a Merkel ending constituted 80% of the epidermal ridges making up the fingerprint patterns. The lack of comparative data from chimpanzees and other apes precludes any definitive conclusions and speaks to the need for additional data.
In conclusion, evidence that the type of grip adopted by the subject in picking up a piece of food differs between hands should not be trivialized in the context of comparative studies of hand use in primates. Few if any studies considered the potential role of grip type in hand use, particularly in wild apes (but see Boesch and Boesch, 1993; Byrne et al., 2001). In fact, it could be argued that the expression of different grasping techniques between hands truly reflects a functional specialization that operates or appears to be neurologically independent of hand preferences. In this sense, the evidence presented here and by others (Byrne and Byrne, 1991; Christel, 1993, 1994; Christel et al., 1998; Hopkins et al., 2002; Tonooka and Matsuzawa, 1995) is arguably the most compelling evidence of a functional specialization in motor skill in nonhuman primates, given the lack of population-level asymmetry in hand preference for simple reaching. Unfortunately, few if any studies systematically attempted to examine asymmetries in motor skill, but rather focused almost entirely on hand preferences in general (Andrews and Rosenblum, 1994, 2001; Costello and Fragaszy, 1988; Rigamonti et al., 1998). We believe there needs to be more research on the association between hand preference and hand skill in nonhuman primates, particularly as it pertains to variation in the morphology of the hand. Conceptually, this will provide for a stronger basis on which to interpret preference data as well as allow for a direct assessment of specialization in motor functions.
Acknowledgments
This research was supported by NIH grants NS-42867, NS-36605, and RR-00165 to the Yerkes National Primate Research Center. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study. We appreciate the assistance of the entire veterinary staff at the Yerkes Center in obtaining fingerprints. Helpful comments and discussions on the relationship between fingerprint patterns and sensory innervation of mechanoreceptors with Drs. Frank Rice and Michel Paré were also appreciated.
Grant sponsor: NIH; Grant numbers: NS-42867, NS-36605, RR-00165.
Literature Cited
- Andrews MW, Rosenblum LA. Automated recording of individual performance and hand preference during joystick-task acquisition in group-living bonnet macaques (Macaca radiata) J Comp Psychol. 1994;103:358–362. doi: 10.1037/0735-7036.108.4.358. [DOI] [PubMed] [Google Scholar]
- Andrews MW, Rosenblum LA. New methodology applied to bonnet macaques (Macaca radiata) to address some contradictory evidence on manual asymmetries in Old World monkeys. J Comp Psychol. 2001;115:1123–1130. doi: 10.1037/0735-7036.115.4.418. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, right, hand, and brain: the right-shift theory. London: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- Annett M. Five tests of hand skill. Cortex. 1992;28:583–600. doi: 10.1016/s0010-9452(13)80229-8. [DOI] [PubMed] [Google Scholar]
- Biegert J. Dermatoglyphics in the chimpanzee. In: Bourne G, editor. The chimpanzee: volume 4: behavior, growth, and pathology of chimpanzees. Baltimore: University Park Press; 1971. pp. 273–324. [Google Scholar]
- Boesch C, Boesch H. Different hand postures for pounding nuts with hammers by wild chimpanzees. In: Preuschoft H, Chivers D, editors. Hands of primates. New York: Springer-Verlag; 1993. pp. 31–43. [Google Scholar]
- Bradshaw J, Rogers LJ. The evolution of lateral asymmetries, language, tool use and intellect. San Diego: Academic Press, Inc; 1993. [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Corp N, Byrne JME. Manual dexterity in the gorilla: bimanual and digit role differentiation in a natural task. Anim Cogn. 2001;4:347–361. doi: 10.1007/s100710100083. [DOI] [PubMed] [Google Scholar]
- Christel MI. Grasping techniques and hand preferences in apes and humans. In: Preuschoft H, Chivers D, editors. Hands of primates. New York: Springer-Verlag; 1993. pp. 91–108. [Google Scholar]
- Christel MI. Catarrhine primates grasping small objects: techniques and hand preferences. In: Anderson J, Roeder J, Thierry B, Herrenschmidt N, editors. Current primatology volume IV: behavioral neuroscience, physiology and reproduction. Strasbourg: Université Louis Pasteur; 1994. pp. 37–49. [Google Scholar]
- Christel MI, Kitzel S, Niemitz C. How precisely do bonobos (Pan paniscus) grasp small objects? Int J Primatol. 1998;19:165–194. [Google Scholar]
- Cummins H, Midlo C. Palmar and plantar epidermal ridge configurations (dermatoglyphics) in European-Americans. Am J Phys Anthropol. 1926;9:471–502. [Google Scholar]
- Cummins H, Spragg SD. Dermatoglyphics in the chimpanzee: description and comparison with man. Hum Biol. 1938;10:457–510. [Google Scholar]
- Costello M, Fragaszy D. Prehension in Cebus and Saimiri: I. Grip type and hand preference. Am J Primatol. 1988;15:235–245. doi: 10.1002/ajp.1350150306. [DOI] [PubMed] [Google Scholar]
- Ettlinger G. Hand preference, ability, and hemispheric specialization: how far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: a distinction between handedness and manual specialization. Psychol Bull. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Holt S. The genetics of dermal ridges. Springfield, IL: Charles C. Thomas; 1968. [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): evidence of a left hemisphere specialization in motor skill. J Exp Psychol Gen. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Jones-Engel LE, Bard KA. Precision grips in young chimpanzees. Am J Primatol. 1996;39:1–15. doi: 10.1002/(SICI)1098-2345(1996)39:1<1::AID-AJP1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Loesch DZ. Quantitative dermatoglyphics: classification, genetics and pathology. Oxford: Oxford University Press; 1983. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav Brain Sci. 1987;10:247–303. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: a meta-analysis of methods. J Hum Evol. 1991;21:425–438. [Google Scholar]
- Marzke MW. Precision grips, hand morphology, and tools. Am J Phys Anthropol. 1997;102:91–110. doi: 10.1002/(SICI)1096-8644(199701)102:1<91::AID-AJPA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mi MP, Budy AM, Rashad MN. Ridge count of finger dermal patterns. Prog Dermatoglyph Res. 1982;84:295–301. [PubMed] [Google Scholar]
- Napier JR, Napier PH. A handbook of living primates: morphology, ecology, and behaviour of nonhuman primates. London: Academic Press; 1967. [Google Scholar]
- Newell-Morris L, Fahrenbruch CC, Yost C. Non-human primate dermatoglyphics: implications for human biomedical research. In: Bartosocas C, editor. Progress in dermatoglyphic research. New York: Alan R. Liss; 1982. pp. 189–202. [PubMed] [Google Scholar]
- Newell-Morris LL, Fahrenbruch CE, Sackett GP. Prenatal psychological stress, dermatoglyphic asymmetry and pregnancy outcome in the pigtailed macaque (Macaca nemestrina) Biol Neonate. 1989;56:61–75. doi: 10.1159/000243104. [DOI] [PubMed] [Google Scholar]
- Paré M, Smith AM, Rice FL. Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J Comp Neurol. 2002;445:347–359. doi: 10.1002/cne.10196. [DOI] [PubMed] [Google Scholar]
- Rigamonti MM, Previde EP, Poli MD, Marchant LF, McGrew WC. Methodology of motor skill and laterality: new test of hand preference in Macaca nemestrina. Cortex. 1998;34:693–705. doi: 10.1016/s0010-9452(08)70773-1. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrews R. Comparative vertebrate lateralization. Oxford: Oxford University Press; 2002. [Google Scholar]
- Schaumann B, Alter M. Dermatoglyphics in medical disorders. New York: Springer-Verlag; 1976. [Google Scholar]
- Tonooka R, Matsuzawa T. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. Int J Primatol. 1995;16:17–34. [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol Psychol. 1980;8:351–359. [Google Scholar]


