Abstract
Faces are one of the most salient classes of stimuli involved in social communication. Three experiments compared face-recognition abilities in chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta). In the face-matching task, the chimpanzees matched identical photographs of conspecifics' faces on Trial 1, and the rhesus monkeys did the same after 4 generalization trials. In the individual-recognition task, the chimpanzees matched 2 different photographs of the same individual after 2 trials, and the rhesus monkeys generalized in fewer than 6 trials. The feature-masking task showed that the eyes were the most important cue for individual recognition. Thus, chimpanzees and rhesus monkeys are able to use facial cues to discriminate unfamiliar conspecifics. Although the rhesus monkeys required many trials to learn the tasks, this is not evidence that faces are not as important social stimuli for them as for the chimpanzees.
The human face holds a special place among visual objects. Any social animal must possess the capacity to differentiate and recognize members of its group and, in humans, the face is the most distinctive attribute for indexing identity reliably. (Sergent & Signoret, 1992, p. 56)
Faces are one of the most important and salient classes of stimuli involved in social communication. On initial inspection, they provide invariant information about age, sex, individual identity, and emotion (Burt & Perrett, 1995; Ekman, 1992; Ekman & Oster, 1979; Izard, 1971; Tranel, Damasio, & Damasio, 1988). In the evolution of primates, there has been an increasing trend toward larger and more complex social groups in which individuals rely less on olfactory cues than on visual cues, such as facial signals, for communication (Andrew, 1963a; Marler, 1965). This trend has been accompanied by an elaboration of the mimetic facial muscles used for the production of facial expressions, resulting in greater variability in the kinds of facial expressions made by individuals of these species (Andrew, 1963b; Huber, 1931). Several facial expressions observed in macaques and chimpanzees, particularly expressions that occur during play and submission (e.g., the relaxed open-mouth face and the bared-teeth display, respectively), are proposed to be homologous with laughter and smiling in humans (Preuschoft, 1992; Preuschoft & van Hooff, 1995). This presents a compelling argument not only for morphological similarities in the evolution of certain facial expressions but also for similarities in the communicative function served by these visual signals.
The ability to use the information present in faces and to discriminatively respond to it has undoubtedly played an important role in the evolution of social animals, particularly social mammals (Andrew, 1963a, 1963b; Brothers, 1990). As groups became larger, the ability to garner social knowledge by recognizing and remembering familiar individuals and their relationships with other group members became highly advantageous (Anderson, 1994; Cheney & Seyfarth, 1990; Hinde, 1976). Individuals do not simply respond to specific social stimuli but rather interact within a fluid social field that is constantly changing depending on the behavior and motivation of others. Therefore, not only must individuals be capable of flexibility in their own social interactions, but they also must be able to monitor the relationships of others to survive in the constantly changing social field (Kummer, 1971).
Although researchers have described many different kinds of social knowledge, most agree that the ability to keep track of conspecifics and their social relationships is critical for survival (Cheney & Seyfarth, 1990; Jolly, 1966). For example, macaques live in large, complex social groups in which daughters' rank is determined according to a strict matrilineal hierarchy (Kawai, 1958). In contrast, chimpanzees live in fission-fusion societies in which absolute group size is large but individuals travel in smaller parties that may join and mingle with others and, at times, reunite into larger units (Goodall, 1971; Nishida, 1979). Among chimpanzees, complex patterns of coalitions and agonistic support have been described by numerous researchers (de Waal, 1982; de Waal & Aureli, 1996; Goodall, 1986). These triadic interactions require not only that individuals recognize and discriminate among group members, most likely by using physical characteristics, but also that they have a detailed understanding of each other's social relationships and use this information to their own advantage (Seyfarth & Cheney, 1988). Therefore, face recognition represents an important social adaptation in nonhuman primates, particularly those that exhibit a broad range of cooperative behaviors such as alliance formation, coalitionary support, and intergroup defense (de Waal, 1982; de Waal & Aureli, 1996; Harcourt, 1992; van Hooff, 1988).
Despite the importance of individual recognition, few empirical studies have examined the cognitive and perceptual mechanisms that underlie the ability of nonhuman primates to process social information from faces alone. The majority of research on individual recognition has focused on the auditory, rather than the visual, domain (Cheney & Seyfarth, 1986; Marler, 1976; Waser, 1977). This is not to say that there is no evidence that faces are important for nonhuman primates. Quite to the contrary, numerous studies on macaque monkeys have demonstrated that social stimuli, including conspecifics' faces and facial expressions, are highly salient and elicit species-typical responses at various stages of development. Macaque monkeys have been shown to perform operant tasks to view videos depicting unfamiliar conspecifics (Andrews & Rosenblum, 1993; Butler, 1961; Plimpton, Swartz, & Rosenblum, 1981; Swartz & Rosenblum, 1980). They respond appropriately to the emotional meaning of threatening conspecifics' facial expressions (Humphrey & Keeble, 1974; Miller, Banks, & Ogawa, 1963; Miller, Caul, & Mirsky, 1967; Redican, Kellicutt, & Mitchell, 1971; Sackett, 1966), and they learn at a young age to avoid looking at the direct gaze of an unfamiliar adult conspecific (Mendelson, Haith, & Goldman-Rakic, 1982). Although these studies demonstrate the perceptual significance of social-facial stimuli for macaque monkeys, comparable data from chimpanzees are glaringly absent. Demonstrating similar face-processing skills in species that are closely related to humans would provide support for the hypothesis that face recognition in primates evolved as a cognitive, behavioral, and possibly even neurological adaptation to aid social communication and social awareness as group size expanded.
This study is one in a series of studies investigating the way in which chimpanzees and rhesus monkeys respond to facial stimuli (Parr & de Waal, 1999; Parr, Dove, & Hopkins, 1998; Parr, Hopkins, & de Waal, 1998; Parr, Winslow, & Hopkins, 1999). It offers an improvement over previous studies in that the same general experimental paradigm (i.e., matching to sample [MTS]) was used to test both species, learning performance was analyzed using strict criteria, the number of training trials is reported, and only high-quality photographs were used. The results of three different experiments are presented. The first task, face matching, required subjects to match a pair of identical faces. The second experiment, individual discrimination, required subjects to discriminate two different photographs of the same individual. The final experiment, feature masking, occluded various facial features in an attempt to identify which feature was the most important to maintain individual recognition.
Because of the importance of conspecific face recognition for chimpanzees and rhesus monkeys, we expected both species to do well on all three tasks, although it was difficult for us to speculate about how their performance might differ. This is due in part to the lack of experimental research in this area, particularly the lack of data on face recognition in chimpanzees, and the paucity of training data provided in previous studies of macaques. Two hypotheses are tentatively provided. The first proposes that if face recognition evolved in primates as an adaptation to the increasing demands of social communication as group size increased, then rhesus monkeys and chimpanzees would be expected to demonstrate similar face-recognition skills because both live in complex, socially demanding groups. If, however, face recognition evolved in combination with the general elaboration in brain size and cognitive functions that accompanied hominoid evolution, then chimpanzees would be expected to show advantages over rhesus monkeys in these tasks.
General Method
Subjects
Data were collected on 5 chimpanzees (Pan troglodytes), 3 males and 2 females between 8 and 9 years of age, and 4 male rhesus monkeys (Macaca mulatta) between 5 and 6 years of age, all housed at the Yerkes Regional Primate Research Center. The chimpanzees were nursery-reared in peer groups and then were moved into permanent social groups (see Bard, 1994, for a description of the rearing process). This rearing procedure provided them with normal peer contact early in their social development. Later, they were housed with different combinations of adult chimpanzees and had considerable exposure to a range of neighbors with which they shared some physical contact through mesh. The rhesus monkeys were mother-reared in social groups for their 1st year of life and have been housed individually with auditory and visual contact with conspecifics since then. The rhesus monkeys were acquired by the Yerkes Regional Primate Research Center in 1996.
All subjects had previous training with a variety of cognitive tasks using the MTS paradigm and the computerized-joystick testing apparatus. No subject had any experience matching social stimuli like faces nor any complex digitized stimuli prior to these studies. The chimpanzees were tested in their home cage and were reinforced with grape juice by the human investigator. The rhesus monkeys were tested in an experimental room in cages that were identical to their home cages using an automated joystick system in which correct responses were automatically rewarded with citrus-flavored pellets (90-mg Noyes; P. J. Noyes Company, Inc., Lancaster, NH).
Stimuli
Face stimuli were taken from the Living Links Stimulus Set, an extensive collection of black-and-white digitized photographs of chimpanzees, rhesus monkeys, and other species.1 The chimpanzee photographs were taken primarily from colonies housed outside of the United States, whereas the rhesus monkey photographs were obtained exclusively from colonies housed at the Yerkes Field Station and the Wisconsin Regional Primate Research Center. This colony information, in combination with extensive records kept at the Yerkes Regional Primate Research Center, made it possible for us to confirm that neither the rhesus monkeys nor the chimpanzees had previously been exposed to any of the individuals depicted in the stimulus photographs.
Photographs depicted only the heads and the faces of males and females of all ages displaying different head positions and gaze orientations. These were then digitized using a desktop scanner at a resolution of 75 dots per inch and saved as 256-grayscale bitmap files. Presentation size of the images ranged from 5.00 to 6.25 cm by 5.00 cm. Whenever possible, photographs were combined to keep the age and the sex of the stimulus individuals consistent within each trial so that performance could not be based on recognizing features specific to these parameters. All external cues in the photographs, such as the presence of objects, caging, other animals, or obvious differences in lighting, were edited from the photographs by homogenizing the background using a standard graphics software package (PhotoShop).
Apparatus and Procedure
Stimuli were presented on a PC-compatible computer mounted on a movable audiovisual cart encased in clear Plexiglas that was wheeled approximately 60 cm away from the subject before the beginning of a testing session. The joystick was mounted on an opaque plastic panel attached to the front of the testing cage so that the stick, which was approximately 2 cm in length, protruded vertically through the mesh and could be manipulated by the subject.
The first 2 experiments, face matching and individual discrimination, were presented to the chimpanzees in a simultaneous MTS format. The last task, feature masking, was performed using a sequential MTS format.2 All three tasks presented to the rhesus monkeys were performed using the sequential MTS format. At the onset of each MTS trial, three facial stimuli, collectively called a stimulus set, appeared simultaneously on the monitor in a triangular configuration on a black background along with a white, cross-shaped cursor (1 × I cm). The stimulus on the top of the configuration represented the sample, or the stimulus to be matched. A response was made by moving the joystick-controlled cursor into contact with one of the two laterally displaced comparison stimuli presented in the bottom corners of the configuration. A correct response occurred when subjects moved the cursor into contact with the comparison stimulus that matched the sample on a predetermined stimulus dimension, such as the identity of the individual depicted in the sample photograph. Correct responses were followed by a food reinforcer and an intertrial interval (ITI) of 3 s. Incorrect responses occurred when subjects moved the cursor into contact with the nonmatching comparison stimulus. Incorrect responses were not rewarded and were followed by an ITI of 5 s. During the ITI, the screen remained black. The position of the correct comparison stimulus varied across trials, with an equivalent number of correct choices located on the right and left sides of the configuration. Stimulus sets were presented in a pseudorandom order until each stimulus set in a task had been seen once, after which the random order presentation continued until testing was terminated.
The procedure was similar for presentations using the sequential MTS format, except that at the beginning of a trial only the sample stimulus and cursor appeared on the monitor. Subjects were required to make an orienting response to the sample stimulus by contacting it with the cursor. After the sample was contacted, it was cleared from the monitor, and the two comparison stimuli appeared in the same locations as those described for the MTS. A correct response was indicated in the same way and was followed by the delivery of a food reinforcer and a 3-s ITI, whereas incorrect responses were not rewarded and were followed by an ITI of 7 s for the chimpanzees and 15 s for the rhesus monkeys. Chimpanzee subjects were given approximately 50 trials each day, 3 days per week, and never more than 100 trials per day. The automated system allowed the rhesus monkeys to complete as many trials as they could while the computer was available, typically 2–3 hr per day, 3 days per week. After completion of testing, the joystick was removed from the cage, and the computer cart was taken away.
Data Analysis
Before testing began, two main performance criteria were established: initial acquisition (i.e., the number of trials required before subjects performed significantly above chance levels) and final performance (i.e., the number of trials required for subjects to reach a final performance level above 80% correct). Final performance was used to ensure that subjects fully understood one task before moving on to the next, more complicated, task. After testing began, it became apparent that the chimpanzees and the rhesus monkeys had very different levels of performance. The rhesus monkeys, for example, required many trials to learn the face-matching task, so their initial acquisition criterion was set at the number of trials required before they performed significantly above chance over a 100-trial block. To assess how well the monkeys understood the task, it was necessary to provide them with a generalization session that compared their relative performance on the original trials with their performance on an equal number of novel trials. Previous studies performed generalization sessions in many different ways, but in these experiments, generalization was performed by adding an equal number of novel stimuli as were provided during the acquisition session and analyzing Trial 1 performance on these novel trials. Final performance for the rhesus monkeys was then assessed as the number of trials required before performance reached above 80% correct for a 100-trial block.
The chimpanzees' performance was significantly above chance within only a few trials, so we determined that they did not require a generalization phase to demonstrate task acquisition. The initial acquisition for the chimpanzees was therefore analyzed as the percentage of correct responses on the first and second exposures, Trim 1 and Trial 2 performance, to each of the stimulus sets in a task. We used binomial z scores to determine whether this performance was significantly above chance on the basis of the number of trials presented. We assessed final performance as the number of trials required for performance to exceed 80% correct.
Experiment 1: Face Matching
Previous studies have reported that macaque monkeys are able to recognize the faces of familiar and unfamiliar conspecifics. Rosenfeld and Van Hoesen (1979), for example, trained rhesus monkeys to discriminate the face of an unfamiliar conspecific from a group of distractor faces. Dittrich (1990) used a similar go/no-go paradigm to train long-tailed macaques to select a target facial expression from a group of four schematic drawings of conspecifics' facial expressions. These studies did not provide very strong support for conspecific face recognition because they required subjects to remember only one target face. The face-matching task examines the ability of chimpanzees and rhesus monkeys to match numerous identical portraits of unfamiliar conspecifics. Although this experiment does not address whether subjects view these faces as representative of specific individuals, it does provide an initial assessment of how quickly and accurately subjects are able to acquire discriminations involving complex digitized facial images.
Stimuli
Twenty-five face-matching stimulus sets were presented to the chimpanzees, with the correct pair of faces consisting of two identical photographs of unfamiliar conspecifics. Eight stimulus sets were presented to the rhesus monkeys during the acquisition phase, with eight novel stimulus sets being added in the generalization phase. In no instance was an individual depicted in the sample stimulus ever repeated in another stimulus set, meaning that all of the individuals represented in these tasks were unique in each trial. Therefore, Experiment 1 required photographs of 50 different chimpanzees and 32 different rhesus monkeys. These numbers are considerably larger than the number of stimuli provided in previous experiments.
Results
Chimpanzees
To assess initial acquisition, we broke the chimpanzee data into the performance on the first and second exposures to each of the 25 stimulus sets. The first exposure represented Trials 1–25, and the second exposure represented Trials 26–50. These 50 trials were typically obtained within a single testing session lasting 1 day. A binomial z-score analysis performed on both the individual and group data revealed that 2 of the 5 subjects and the group as a whole performed significantly above chance on the first exposure to the face-matching discriminations. We should emphasize that this is above-chance Trial 1 performance by subjects that had no prior exposure to any discrimination involving a complex digitized image. By the second exposure, which was performed during the same initial day of testing, every individual subject performed significantly above chance. Table 1 lists the percentage of correct responses and the binomial z scores for each subject's performance and the group data on the first and second exposures to this task.
Table 1.
Percentage of Correct Responses and Binomial z Scores for Chimpanzees on the First and Second Exposures to the 25 Stimulus Sets in the Face-Matching Task in Experiment 1
| First exposure (TriMs 1–25) |
Second exposure (TriMs 26–50) |
|||
|---|---|---|---|---|
| Subject | % correct | z score | % correct | z score |
| Jarred | 60 | 1.00 | 80 | 3.00** |
| Katrina | 76 | 2.60** | 76 | 2.60** |
| Kengee | 60 | 1.00 | 84 | 3.40** |
| Lamar | 56 | 0.60 | 76 | 2.60** |
| Scott | 76 | 2.60** | 92 | 4.20** |
| Group results | 66 | 3.49** | 82 | 7.07** |
p < .01.
Final performance was then assessed in terms of the number of trials required before subjects performed more than 80% correct. The number of trials needed before performance reached 80% correct and the percentage at which this criterion was reached were as follows: for Jarred, 195 trials, 85%; for Katrina, 73 trials, 80%; for Kengee, 238 trials, 87%; for Lamar, 232 trials, 81%; and for Scott, 76 trials, 89%. With approximately 50 trials being given each testing session, this represented between 2 and 5 days of testing.
Rhesus monkeys
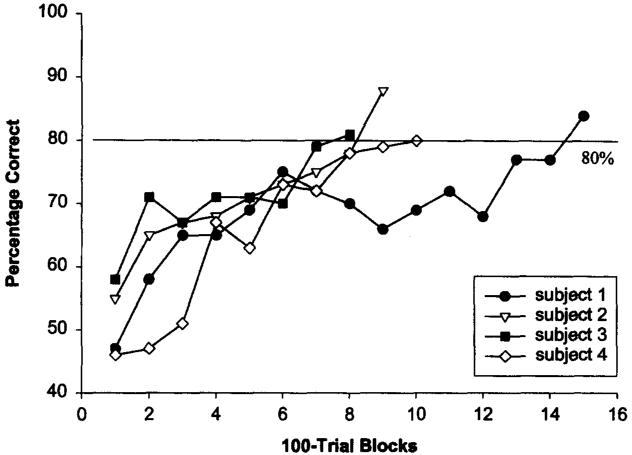
A binomial z score determined that 60% or greater correct represented performance that was significantly above chance for a 100-trial block. The number of trials required before subjects reached this initial acquisition criterion and their percentage of correct responses on reaching it were as follows: for Subject 1, 1,400 trials, 67%; for Subject 2, 700 trials, 67%; for Subject 3, 1,000 trials, 61%; and for Subject 4, 1,300 trials, 61%. Because the rhesus monkeys worked on an automated system, they were able to complete as many trials as they could while the computer was available. Many of them performed more than 1,000 trials in the 1st week of testing, comparable to the duration of time required for the chimpanzees to acquire the task. Before moving on to the generalization phase, subjects were required to meet a final performance criterion of greater than or equal to 80% correct for 100 trials. The number of trials needed to reach this criterion and the percentage of correct responses for the 100-trial block were as follows: for Subject 1, 3,100 trials, 82%; for Subject 2, 1,100 trials, 85%; for Subject 3, 2,100 trials, 87%; and for Subject 4, 3,300 trials, 80% (see Figure 1).
Figure 1.
The mean acquisition data for rhesus monkeys in the face-matching task in Experiment 1. Data are presented as the percentage of correct responses over each 100-trial block. The final performance criterion of 80% correct is marked.
The subjects were then presented with a generalization phase that combined 8 novel trials with the 8 originals, The relative performance on the old and new trials was then compared over blocks of 16 trials, or 2 exposures to each stimulus set, for ease of analysis. The subjects performed significantly better on the original face-matching trials than on the novel trials after 16 exposures, t(3) = 3.39, p < .05, but no difference was found after 32 trials, t(3) = 2.56, ns. Individually, 3 of the 4 subjects performed significantly above chance on the novel discriminations after two blocks of 16 trials. This represents only 4 exposures to each of the 8 novel discriminations. One subject (Subject l) failed to perform significantly above chance on the novel discriminations after 50 exposures (M = 53%, z = 0.43, ns) but maintained above-chance performance on the original discriminations (M = 78%, z = 4.06,p < .01). Table 2 lists the percentage of correct responses and the binomial z scores for each subject's performance after 16, 32, and 50 exposures to the novel generalization trials.
Table 2.
Percentage of Correct Responses and Binomial z Scores for Rhesus Monkeys After 16, 32, and 50 Exposures to the Generalization Phase of the Face-Matching Task in Experiment 1
| 16 trials |
32 trials |
50 trials |
||||
|---|---|---|---|---|---|---|
| Subject | % correct | z score | % correct | z score | % correct | z score |
| Subject 1 | 44 | −0.50 | 50 | 0.00 | 53 | 0.43 |
| Subject 2 | 69 | 1.50 | 66 | 1.76* | 65 | 2.22** |
| Subject 3 | 69 | 1.50 | 81 | 3.53** | 76 | 3.67** |
| Subject 4 | 75 | 2.00** | 72 | 2.47** | 71 | 3.05** |
| Group results | 64 | 2.25** | 67 | 3.89** | 67 | 4.71** |
p < .05, one-tailed.
p < .01.
Discussion
The face-matching task confirmed that chimpanzees and rhesus monkeys were able to match the faces of unfamiliar conspecifics and that these faces represent salient, discriminable stimuli even when they are presented as two-dimensional black-and-white images on a computer screen. Chimpanzees performed significantly above chance on their first exposure to the 25 stimulus sets presented to them, having never before seen digitized stimuli. The rhesus monkeys required many trials to learn the face-matching task (i.e., an average of 1,000 trials), but when they were presented with novel faces in a generalization session, their performance was significantly above chance after only 32 trials, or four exposures to each of the eight novel face-matching discriminations. These results suggest that once the rhesus monkeys had acquired the task, they had little difficulty discriminating photographs of novel individuals. This generalization performance is strong relative to that reported in previous studies. This experiment supports the conclusion that chimpanzees and rhesus monkeys possess the cognitive skills necessary to learn discriminations involving the faces of unfamiliar conspecifics even though they were presented as digitized black-and-white static images.
A direct comparison between face-recognition performance in this experiment and how individuals learn to recognize conspecifics in the wild is not possible, although we should stress that 1,000 trials does not translate into an unusually long exposure duration to these stimuli. Nonhuman primates, for example, work rapidly on the joystick task; they do not pause to consider the correct answer, as most humans do. Because eight different unfamiliar individuals were presented in this task, the subjects saw each individual just under 125 times before reaching the acquisition criterion. The sample image was present for approximately 3 s before the orienting response was typically made. Therefore, the total exposure duration was just under 6.5 min per individual. This duration was not so long as to make the data incomparable to how rapidly individuals in a wild troop may learn to recognize one another or how the subjects learn to discriminate individuals that are familiar group mates. Realistically, this seems like a very short amount of time to process and remember two-dimensional black-and-white images of unfamiliar conspecifics presented on a computer screen and suggests that face recognition is a prominent component of the natural behavioral repertoire of nonhuman primates.
Experiment 2: Individual Discrimination
The previous experiment demonstrated the ability of chimpanzees and rhesus monkeys to discriminate between two identical photographs of unfamiliar conspecifics' faces, but it did not address whether the subjects discriminated these stimuli as faces per se. Only a few studies have attempted to demonstrate individual recognition in nonhuman primates, and all of these studies addressed this basic skill in strikingly different ways. Dasser (1988) reported evidence for kin recognition by using facial cues in two long-tailed macaques after 1 year of training, each using a different paradigm, simultaneous discrimination or MTS. These monkeys were able to select portraits of familiar mothers with their offspring but only when the portraits had been taken recently.3 Recent data from our own lab suggest that chimpanzees spontaneously match the faces of unfamiliar mother and son pairs but not mother and daughter pairs nor unrelated individuals (Parr & de Waal, 1999). That study provided the first evidence for kin recognition in a primate species that is based on purely visual cues (i.e., phenotypic matching) and is independent of early association because the experimental subjects had no prior experience or familiarity with the individuals whose faces were presented.
Two studies have reported that chimpanzees are able to recognize familiar individuals by using a form of symbolic language, namely, American Sign Language (ASL) or lexigrams. Bauer and Philip (1983) demonstrated the ability of three chimpanzees to use ASL to perform cross-modal identification of familiar individuals by using facial portraits and vocal recordings. Similarly, one chimpanzee succeeded in associating lexical symbols with photographs of familiar chimpanzees and humans (Itakura, 1992; Tomonaga, Itakura, & Matsuzawa, 1993). These results highlight that chimpanzees have a keen awareness for their familiar social companions and that they can be trained to use abstract labels to represent the identity of these individuals. Finally, Boysen and Bemtson (1989) measured the heart rate of one chimpanzee while she viewed photographs representing three categories of chimpanzees: friendly, aggressive, and unfamiliar. Heart rate was acceleratory when the subject viewed the chimpanzee that was repeatedly hostile toward her, suggestive of a defensive response. Cardiac deceleration was observed when the subject viewed a photograph of the unfamiliar chimpanzee, reflecting an orienting response to a novel individual. Neutral responses were observed in response to the friendly chimpanzee. The physiological pattern of response made by this subject reflected her relationship history with the individuals depicted in the photographs. Thus, this study demonstrated several different levels of conspecific recognition by using an objective methodology and elegant paradigm. This is an exciting finding because it provides initial evidence not only that faces provide information about individual identity but also that chimpanzees associate strong emotional meaning toward conspecifics.
In Experiment 2, individual discrimination, we examined the degree to which chimpanzees and rhesus monkeys were able to recognize unfamiliar individuals by presenting them with the task of matching two different photographs of the same individual. The subjects were, therefore, unable to rely on the perceptual features of the photographs, like shading, symmetry, and luminance, because these features varied across the photographs.
Stimuli
The chimpanzees were presented with 14 stimulus sets in which the correct pair of faces was 2 different photographs of the same individual. These were presented along with 10 novel face-matching stimulus sets as control trials to ensure that the subjects were still performing these discriminations according to the matching concept. Therefore, 42 different photographs were needed for this task, that is, 28 photographs of 14 different individuals for the matching pair and 14 additional photographs of novel individuals as the nonmatching comparisons.
The acquisition phase of the rhesus monkey individual-discrimination task consisted of 20 stimulus sets, 15 individual-discrimination sets plus 5 of the original face-matching sets, which were included to ensure that the matching performance was maintained. Ten novel individual-discrimination stimulus sets were later added to these 20 for the generalization phase. Therefore, the acquisition phase required photographs of 30 different rhesus monkeys but 45 different photographs. In the generalization phase, an additional 30 photographs were needed from 20 different individuals. Figure 2 illustrates an example of a rhesus monkey individual-discrimination trial.
Figure 2.
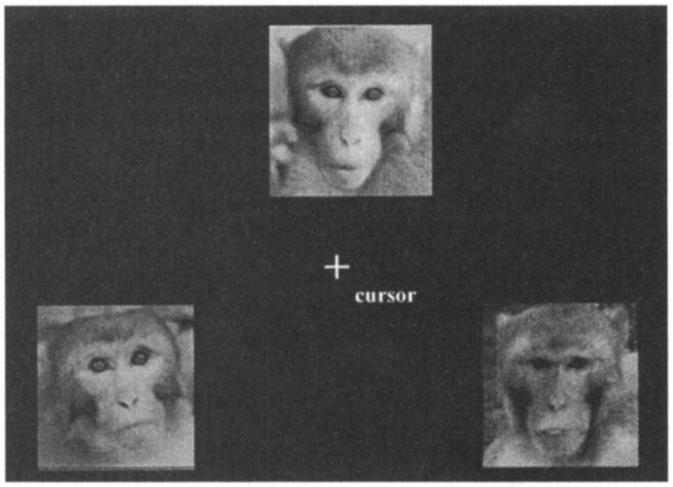
An example of a rhesus monkey individual-discrimination trial presented in the individual-discrimination task in Experiment 2. The correct choice is the individual presented on the bottom left.
As in the face-matching task, the photographs were combined with different head positions and eye gaze orientations. In several of these stimulus sets, the age of the individuals varied between the time the sample and target photographs were taken, although in no stimulus set did the correct pair combine photographs of individuals when they were infants and adults. According to this arrangement, correct matching could be accomplished only by recognizing the stimulus individual's physiognomic features, not features of the photographs themselves, because three different photographs were used in every stimulus set.
Results
Chimpanzees
We first analyzed the acquisition data in terms of each subject's performance on the first and second exposures to the 10 novel face-matching control trials and the 14 individual-discrimination trials. No individual subject's performance was above chance after the first exposure to the novel face-matching trials partly because of the small number of stimulus sets presented: Performance was required to exceed 90% to be significantly above chance for only 10 discriminations (z = 2.53, p < .05). The group performance, however, was 64% and 72% for Trial 1 and Trial 2, respectively. The group performance was significantly above chance on Trial 1 (z = 1.98, p < .05), indicating that the subjects were conforming to the general MTS rule.
The mean performance on the first and second exposures to the individual-discrimination trials was 59% and 71%, respectively. Neither the group nor any individual subject performed significantly above chance on Trial 1. By the second exposure, however, 2 of the 5 subjects and the group performed these discriminations significantly above chance. Emphasis should be placed on the fact that no additional training was given to subjects to teach them the new dimension of matching. Experiment 2 was presented directly after completion of Experiment 1. Table 3 lists the percentage of correct responses and the binomial z scores for each subject's Trial 1 and Trial 2 performance and the group data on each of the 14 individual-discrimination trials.
Table 3.
Percentage of Correct Responses and Binomial z Scores for Chimpanzees on the First and Second Exposures to Each of the 14 Individual-Discrimination Stimulus Sets in the Individual-Discrimination Task in Experiment 2
| First exposure (Trials 1–14) |
Second exposure (Trials 15–28) |
|||
|---|---|---|---|---|
| Subject | % correct | z score | % correct | z score |
| Jarred | 43 | −0.53 | 86 | 2.67** |
| Katrina | 57 | 0.53 | 71 | 1.60 |
| Kengee | 64 | 1.07 | 64 | 1.07 |
| Lamar | 57 | 0.53 | 50 | 0.00 |
| Scott | 71 | 1.60 | 86 | 2.67** |
| Group results | 59 | 1.44 | 71 | 3.59** |
p < .01.
The task paradigm was switched from MTS to sequential MTS during the final acquisition phase of this task. The number of trials required for the subjects to perform above 80% and the percentage at which this criterion was reached were as follows: for Jarred, 30 trials, 80%; for Katrina, 204 trials, 82%; for Kengee, 28 trials, 82%; for Lamar, 28 trials, 86%; and for Scott, 209 trials, 85%. As a side comment, we should note that Scott and Katrina had changed home cages before the sequential MTS individual-recognition task was administered and often were distracted by events made novel by their new environment. This may be one reason for the discrepancy in their final acquisition performance compared with the rest of the subjects, all of whom met the final performance criterion within the 1st day of testing.4
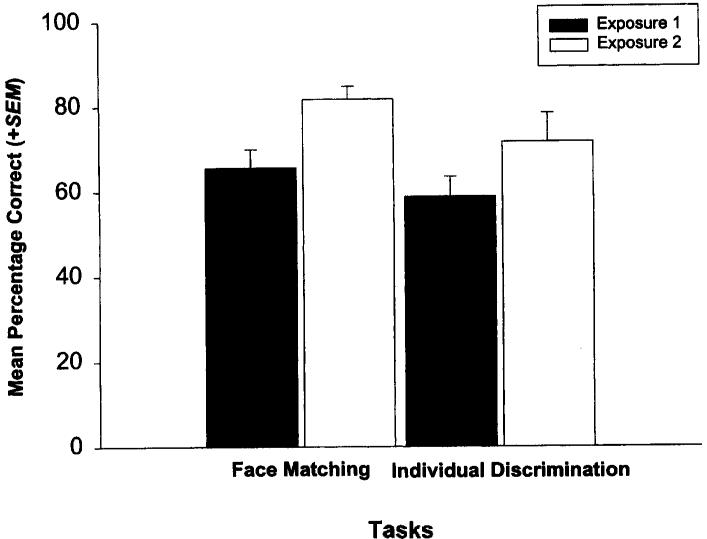
Figure 3 illustrates the mean performance on the first and second exposures to the tasks in Experiments 1 and 2, face matching and individual discrimination, respectively. There was no significant difference between the performance on the first and second exposures to the stimulus discriminations in either of these experiments, indicating that the subjects responded to them in similar ways with no additional training, t(1) = 5.48, ns.
Figure 3.
A comparison between the mean performance (+SEM) of chimpanzees on the first and second exposures to the tasks in Experiments 1 and 2, face matching and individual discrimination, respectively.
Rhesus monkeys
Individually, the acquisition data for the rhesus monkey individual-discrimination trials were analyzed according to each subject's performance over blocks of 100 trials. The number of trials needed before subjects performed significantly above chance for a 100-trial block and their percentage of correct responses on reaching this criterion were as follows: for Subject 1, 300 trials, 65%; for Subject 2, 200 trials, 65%; for Subject 3, 200 trials, 71%; and for Subject 4,400 trials, 67%. Subjects required significantly fewer trials to reach the initial acquisition criterion on the individual-discrimination task than on the original face-matching task in Experiment 1, t(3) = 6.60, p < .01.
Before moving on to the generalization phase, the subjects were required to reach the second final performance criterion of greater than 80% correct for 100 trials. The number of trials needed to reach this criterion and the percentage of correct responses on reaching it were as follows: for Subject 1, 1,500 trials, 84%; for Subject 2, 900 trials, 88%; for Subject 3, 800 trials, 81%; and for Subject 4, 1,000 trials, 80%. Figure 4 illustrates the acquisition data for the rhesus monkey individual-discrimination task in terms of the mean performance over 100-trial blocks. The generalization phase analyzed subjects' performance on the 10 novel individual-discrimination trials according to 20-trial blocks, with only two exposures to each of the novel trials. Performance was required to be at least 70% (14 out of 20, one-tailed) to be significantly above chance for a single block of 20 trials. The number of 20-trial blocks that each subject required before its performance was significantly above chance and the percentage of correct responses once the criterion was reached were as follows: Two subjects generalized their performance to the novel individual-discrimination trials significantly above chance on the first 20-trial block (95% and 85%); 1 subject required 40 trials (70%), or two blocks; and 1 subject required 140 trials (70%). As a group, the subjects required significantly fewer trials to reach criterion on the novel individual-discrimination trials than on the initial acquisition trials, again indicating strong generalization performance once the initial task was learned, t(3) = 7.47, p < .05.
Figure 4.
The mean acquisition data for rhesus monkeys in Experiment 2. Data are presented as the percentage of correct responses over each 100-trial block. The final performance criterion of 80% correct is marked.
Discussion
The chimpanzees performed discriminations of 14 unfamiliar conspecifics significantly above chance on their second exposure, or Trial 2 performance. Because they did not receive any additional gaining on this task after the conclusion of Experiment 1, it appeared as though one exposure to the novel stimuli was needed before the subjects recognized that the dimension of matching had changed from one involving identical photographs to one involving two different photographs of the same individual. This is the first such demonstration of individual recognition in chimpanzees using the photographs of unfamiliar conspecifics. Thus, these data support the hypothesis that chimpanzees are able to use facial features, not features of the photographs, to make discriminations involving individual identity. This is not to say that photographic features could not be used to make these discriminations. However, this remains an unlikely explanation for these experimental results primarily because of the fact that the chimpanzees' performance was significantly above chance on Trial 2. This makes explanations for performance based on subjects learning the reinforcement contingencies associated with the perceptual features of each of the 28 photographs highly unlikely. A previous study involving the same subjects demonstrated that performance on stimulus discriminations that randomly combined three unfamiliar, unrelated individuals never exceeded above-chance levels, even after several hundred trials (Parr & de Waal, 1999). These data demonstrate that even if subjects could use photographic cues as discriminatory features, they are certainly not learned or used within two stimulus presentations.
As in Experiment 1, the rhesus monkeys required considerable training to learn the individual-discrimination task, although they required significantly fewer trials than they did on the face-matching task (i.e., an average of 1,050 trials compared with 2,400 trials, respectively) before they reached the final performance criterion. It appeared as though their understanding of the MTS task became stronger in the second experiment, as would be expected with practice. During the generalization phase, 2 subjects performed significantly above chance after only 20 trials, or 2 exposures to the 10 novel stimulus sets. One subject required 4 exposures, and 1 subject required 14 exposures, or 140 trials, still significantly fewer trials than this subject needed to acquire the task. Rhesus monkeys, like chimpanzees, appear capable of recognizing unfamiliar individuals by using facial cues, even when these stimuli are presented as two-dimensional images using a computerized task.
These data appear to be quite strong when they are compared with other well-known studies in the literature. Dasser (1988), for example, provided more than a year of training to 1 subject before presenting the subject with 22 transfer trials in an MTS task. The generalization performance of this subject was quite strong, but the transfer trials were presented individually as probe trials mixed with the training trials over an extended period of time (i.e., 11 months). This is not comparable to the way in which generalization trials were presented in the present experiments, in which novel trials were added as a group to the acquisition trials and the data were then analyzed in terms of Trial 1 performance on the novel faces.
Experiment 3: Feature Masking
Human studies have suggested that the eyes are the most important and memorable facial feature involved in individual recognition (McKelvie, 1976). Studies of nonhuman primates also have suggested that the eyes receive more visual attention than other areas of the face and body (Keating & Keating, 1993) or that individuals prefer to look at unaltered faces compared with faces in which the eyes are masked (Kyes & Candland, 1987). These results were confirmed in a study that measured the visual scanning patterns of four rhesus monkeys while viewing still faces and videos of conspecifics' faces (Nahm, Perret, Amaral, & Albright, 1997). They concluded that the eyes were the most salient facial feature, regardless of whether the stimuli were still or moving images.
In Experiment 3, we presented faces with three different types of feature masking—the eyes, the mouth, and the eyes and the mouth together—to determine which facial feature was most important for maintaining performance on the individual-recognition task. Of interest was not so much how subjects learn discriminations when various features are occluded, because alternative strategies for performance, ones other than individual recognition, may result, making it difficult to demonstrate the contribution of specific features for individual recognition per se. Rather, we designed this experiment to examine the role of each feature for maintaining individual recognition once it had been acquired. We expected that, as in the previous studies, the greatest impairments in individual recognition would occur when the eyes or the eyes and the mouth together were masked.
Stimuli
Twenty stimulus sets were used in the chimpanzee and rhesus monkey feature-masking tasks. These consisted of the original individual-discrimination stimulus sets described in Experiment 2. Therefore, the subjects were already performing at a fairly high level on these discriminations, and the contribution of each feature for maintaining this performance could be addressed by using a masking procedure. The 14 stimulus sets from the chimpanzee individual-discrimination task were combined with six control trials taken from the original face-matching task, and the 15 rhesus monkey individual-discrimination stimulus sets were combined with five control trials taken from the original rhesus monkey face-matching task. Because the correct pair of stimuli was two different photographs of the same individual, matching required the recognition of specific facial features of that individual, not features of the photograph that were visible behind the masking. In addition, the position of the correct response was reversed from its original presentation side.
Masking was performed on the sample photographs by placing a gray rectangle over the particular feature by using the PhotoFinish graphics software package. The comparison faces remained unaltered. In 5 of the original 14 individual-discrimination trials, the eyes were masked; in 5 trials, the mouth was masked; and in 4 trials, the eyes and the mouth were masked together. The eyes were masked so that a grayish-colored rectangle extended from the brow ridge to the top of the nose, concealing the sides of the head. In the mouth condition, both the mouth and the lower portion of the nose were completely covered. Finally, in the last four sample photographs, masking was performed by using two gray blocks covering the areas of the eyes and the mouth according to the aforementioned criteria.
The rhesus monkey sample photographs were masked in a similar way. Five stimulus sets contained sample photographs in which the eyes were masked with a gray rectangle that extended to the sides of the head over the eyebrow region but not occluding the nose. Five sample photographs were masked so that a gray rectangle covered the mouth of the individual, beginning near the nose and extending to the sides of the cheeks but, when possible, not covering the tip of the chin. Masking of the eyes and the mouth was performed on another five sample photographs following the same procedure.
Results
Chimpanzees
Each subject received a fixed total of 150 trials on the feature-masking task. The mean performance levels for each category of facial masking were as follows: eyes masked, 66%; mouth masked, 74%; eyes and mouth masked, 71%; and 81% on the unaltered face-matching control trials. Binomial z scores determined that the group's performance for each masking type was significantly above chance (z = 4.35, p < .01; z = 6.71, p < .01; z = 5.07, p < .01; and z = 9.26, p < .01, respectively), indicating that masking facial features was not sufficient to significantly impair individual recognition in chimpanzees. A paired t test (two-tailed) compared the performance on each masking type with the unaltered control trials (i.e., face-matching trials) to determine which type of feature significantly impaired individual recognition when masked. This analysis revealed that only when the eyes alone were masked did performance become significantly impaired, t(4) = −2.82, p < .05. No significant differences were found between the control trials and the other two feature-masking types: the mouth and both the eyes and the mouth.
Rhesus monkeys
As with the chimpanzees, the rhesus monkeys received 150 trials on the feature-masking task. The mean performance levels for each category of facial masking were as follows: eyes masked, 85%; mouth masked, 93%; eyes and mouth masked, 81%; and 97% on the unaltered face-matching control trials. Binomial z scores determined that the group's performance for each of the four trial types was significantly above chance (z = 8.50, p < .01; z = 10.53, p < .01; z = 7.57, p < .01, and z = 11.64, p < .01, respectively). As with the chimpanzees, masking various facial features did not impair performance significantly below chance levels. Paired t tests (two-tailed) were then performed to determine which masking type significantly impaired performance compared with the unaltered face-matching control trials. This analysis revealed that only when both the eyes and the mouth were masked together was performance significantly impaired from the control trials, t(3) = −3.43, p < .05. None of the other comparisons reached significance.
Discussion
The general pattern of results from the feature-masking experiment confirms the importance of the eyes for both chimpanzees and rhesus monkeys for maintaining individual recognition. Rhesus monkeys performed significantly below control trials when the eyes and the mouth were masked together, whereas the chimpanzees performed significantly below the control trials only when the eyes alone were masked and showed no significant impairment when the eyes and the mouth were masked together. Differences between the performance of chimpanzees and rhesus monkeys may simply reflect variability due to small sample sizes, or they may reflect general species differences in the cues used by chimpanzees and rhesus monkeys for individual recognition. Additional studies are needed to fully understand the role of individual facial features and the characteristics of these features for the recognition of unfamiliar conspecifics.
General Discussion
These experiments demonstrated that chimpanzees and rhesus monkeys responded to faces as salient and discriminable stimuli, despite the fact that only the faces of unfamiliar individuals were used, and these were presented as two-dimensional black-and-white static images. Chimpanzees accurately matched identical photographs of unfamiliar conspecifics' faces significantly above chance on their first exposure to these stimuli, eliminating learning based on reinforcement history as a likely explanation for their performance. Similar Trial 1 performance has never been reported in previous studies of nonhuman primate face recognition. Rhesus monkeys generalized their performance to an equal number of novel trials after only four exposures, demonstrating strong generalization performance compared with previous literature on face processing in this genus. Previous studies typically did not report the number of trials required for subjects to learn the specific task. Even when the data from generalization trials are reported, the Trial 1 performance is often excluded, making data interpretation difficult and raising doubts as to the strength of the findings. It is unclear why training data, or first-trial generalization data, are not published, especially because the way in which subjects learn a task may have important implications for how well it is understood. Even though the rhesus monkeys required an average of 1,000 trials before they performed above chance on the face-matching task, these trials were completed within a few testing sessions.
When presented with two different photographs of unfamiliar individuals, the chimpanzees performed significantly above chance after only two exposures to the stimuli. This finding again leads strongly away from explanations for performance based on learning the reinforcement contingencies associated with each trial, in addition to explanations that subjects may have been using subtle features of the photographs, like luminance or contrast, as discriminatory cues. The rhesus monkeys performed many trials before they acquired the individual discriminations but, nonetheless, demonstrated strong generalization performance, with 2 subjects performing significantly above chance after only two exposures to the novel presentations. Both species, therefore, demonstrated an ability to use facial cues to discriminate unfamiliar conspecifics and not cues pertaining to the photographs themselves, because the correct pairs involved two different photographs. This is not to say that perceptual features like luminance, contrast, and symmetry are unimportant for individual recognition, and future studies should examine the contribution of each of these features to nonhuman primate face-discrimination abilities.
Masking individual facial features, and combinations of features, revealed a potentially important difference between the way in which chimpanzees and rhesus monkeys processed these digital images. Chimpanzees performed significantly worse than they did on control trials when the eyes alone were masked, but the performance of rhesus monkeys was impaired only when the eyes and the mouth were masked together. The performance of the rhesus monkeys was significantly lower than their performance on the control trials only when the greatest number of features was masked and, therefore, may represent an additive effect in the quantity of information that is necessary for accurate recognition. The conclusion of the feature-masking experiment should be that covering the mouth alone does not alter performance in either species but that the eyes play an important role in individual recognition, as they do in humans. This phenomenon should be investigated more thoroughly by using both familiar and unfamiliar individuals as stimulus materials.
These data demonstrate not only that faces represent a salient class of stimuli for both chimpanzees and rhesus monkeys but also that individuals process them in meaningful ways when they are presented using a computerized testing paradigm. Thus, the joystick testing paradigm appears to be an ideal format for assessing cognitive and social issues of a comparative nature (Washburn & Rumbaugh, 1992). This paradigm has already been used to examine the ability of chimpanzees and rhesus monkeys to discriminate among pairs of related individuals (i.e., mothers and off-spring), to categorize their own facial expressions, and to demonstrate the importance of orientation for face recognition (Parr & de Waal, 1999; Parr, Dove, & Hopkins, 1998; Parr, Hopkins, & de Waal, 1998; Parr et al., 1999).
Differences in the way in which chimpanzees and rhesus monkeys perform on these tasks should not be concluded as evidence that chimpanzees are simply better than rhesus monkeys at using facial cues to recognize conspecifics. Previously cited were numerous studies demonstrating that faces and facial expressions represent salient and socially significant categories of stimuli for macaques, which are capable of eliciting species-typical responses. In addition, it has been argued that individual recognition represents an important social adaptation for nonhuman primates that contributes to maintaining group cohesion and acquiring social knowledge. Three different perspectives are taken in an effort to provide a framework for explaining why the chimpanzees and the rhesus monkeys differed in their ability to process information about individual identity from facial stimuli. First, chimpanzees may simply be superior to rhesus monkeys in their ability to perform computerized-joystick tasks, or any experimental task that requires subjects to demonstrate knowledge in a captive setting. These advantages may include differences in the motivation of subjects to perform tasks, interact with human experimenters, comprehend task demands, or manipulate the joystick to select correct responses. Previous studies, however, have demonstrated no overall differences in the ability of chimpanzees and rhesus monkeys to learn to use the computerized-joystick testing system, although the chimpanzees were reportedly more dexterous and produced more elaborate motor responses than the rhesus monkeys (Hopkins, Washburn, & Hyatt, 1996). Therefore, any differences in the face-recognition abilities of rhesus monkeys and chimpanzees reported in these experiments are not likely due to fundamental differences in the way in which chimpanzees and rhesus monkeys acquire joystick-based tasks or the extent to which they understand tasks presented using a computerized paradigm. In addition, there was no evidence of any prominent differences in the motivation of the subjects to perform the joystick tasks, because performance of the subjects was strictly voluntary.
A second possible explanation may be related to species differences in social organization and behavior, or the way in which facial information is obtained and used. Previous studies, for example, have demonstrated that rhesus monkeys perceive direct stares as threatening and learn at a very early age to avoid the direct gaze of conspecifics (Hinde & Rowell, 1962; Mendelson et al., 1982). Therefore, it may not be as important, or adaptive, for rhesus monkeys to rely heavily on the eyes as the most prominent cue for recognizing conspecifics. It would be to their advantage to rely on other cues, or a combination of cues, for individual recognition and to avoid the need for prolonged eye contact. In contrast, chimpanzees exhibit no such inhibition and gaze directly into the eyes of conspecifics and human observers, often with a communicative intent (Leavens & Hopkins, 1998). The eyes appear to provide chimpanzees with the most reliable cue for gaining relevant information about an individual's identity, at least as demonstrated in these experiments. Additional studies should examine the extent to which other facial features, or combinations of features, are involved in individual recognition, including the role of direct and averted gaze, scrambled faces, and faces in which spatial frequency has been altered.
Finally, chimpanzees may possess more generally advanced cognitive abilities than rhesus monkeys, which may provide them with advantages in understanding the relationship between the stimuli presented in these experiments and decreasing the time needed to acquire the tasks. There is considerable evidence from both experimental studies and observations of naturally occurring behavior that support the conclusion that chimpanzees are superior to most monkey species in their cognitive abilities. Chimpanzees, for example, make and use tools in the wild and exhibit elements of culture (Boesch & Boesch, 1984; Goodall, 1971; McGrew, 1992). They not only acquire forms of language through the use of lexigrams and ASL but also construct these symbols in logical, meaningful, and novel ways (Fouts, Chown, & Goodin, 1976; Matsuzawa, 1985, 1989; Savage-Rumbaugh, McDonald, Sevcik, Hopkins, & Rubert, 1986). Chimpanzees perceive the difference between concrete and abstract same–different relationships (Oden, Thompson, & Premack, 1990) and show better transfer, or generalization performance, in MTS tasks than do New World or Old World monkeys (D'Amato, Salmon, & Colombo, 1985; Nissen, Blum, & Blum, 1948; Oden, Thompson, & Premack, 1988). Chimpanzees also have been shown to pass the mark test of mirror self-recognition, whereas monkeys do not (Gallup, 1970). Indeed, in the present study, the chimpanzees acquired the discriminations so quickly as to make generalization trials unnecessary (i.e., Trial 1 performance).
Although research suggests that chimpanzees may have more elaborate cognitive skills than rhesus monkeys, no conclusions can be drawn about the involvement of specific neural mechanisms that underlie these abilities. Studies on relative brain size have demonstrated that chimpanzees have the largest brain per body size of any Old World or hominoid species apart from humans. In addition, recent imaging studies have documented the presence of elaborated brain structures in chimpanzees and bonobos, but not rhesus monkeys or capuchins, that appear to be morphologically homologous to functional areas of the human brain (Gannon, Holloway, Broadfield, & Braun, 1998; Hopkins, Marino, Rilling, & MacGregor, 1998). Are the differences in face processing reported in these experiments the result of a general improvement in the cognitive-processing skills of the chimpanzee brain? Can these skills be identified as dependent on specific systems or pathways, and is there comparative evidence that these pathways are less developed in macaques than in chimpanzees and humans? Future studies should be performed that combine modern imaging techniques (e.g., positron emission tomography) with cognitive tasks similar to the ones presented in these experiments to elaborate on the neural mechanisms underlying face processing in these two species. Such a collaboration would provide the most clearly detailed information yet available concerning the comparative, cognitive, and neural mechanisms underlying face recognition, one of the most basic yet critical skills involved in social communication for all primates, including humans.
Acknowledgments
This investigation was supported by Grant RR-00165 from the National Institutes of Health/National Center for Research Resources, by Grant NINDS-29574, and by Grant R01-RR09797. The Yerkes Regional Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
We thank Kim Bard, Kathy Gardner, and Elizabeth Hearn for additional photographic stimuli; Frank Kiernan for photographic assistance; and the animal care staff at the Yerkes Regional Primate Research Center. Helpful comments on earlier versions of this article were provided by Michelle Berger, Kate Egan, and Agnès Lacreuse.
Footnotes
Portions of this article were presented at the International Primatological Society Joint Congress, August 1996, Madison, Wisconsin.
Inquiries and purchase requests for the Living Links Stimulus Set should be sent to www.emory.edu/LIVING_LINKS.
The stimulus sets used in these experiments are available from Living Links (www.emory.edu/LIVING_LINKS).
The reasons for switching to the sequential MTS format were unrelated to the experimental questions in this study. The chimpanzees were then given the face-matching task in the sequential MTS format, and their mean performance was 82% after 60 trials (Parr, 1998).
Dasser did not report the number of training trials needed to reach this acquisition performance. Although it did take an average of 1,000 trials for the rhesus monkeys to acquire the face-matching task, this time period represented only a few weeks of training.
Previous studies have discounted data acquired when subjects were distracted by external events (e.g., Dasser, 1988). We, however, do not feel as though this decision can be made objectively, so we included all training data.
Contributor Information
Lisa A. Parr, Living Links Center, Yerkes Regional Primate Research Center, and Department of Psychology, Emory University
James T. Winslow, Division of Psychobiology, Yerkes Regional Primate Research Center, and Medical School, Emory University
William D. Hopkins, Living Links Center, Yerkes Regional Primate Research Center, Emory University and Department of Psychology, Berry College
Frans B. M. de Waal, Living Links Center, Yerkes Regional Primate Research Center, and Department of Psychology, Emory University
References
- Anderson JR. Valeur ethologique des visages et des mimiques chez les primates non humains [Ethological value of faces and mimics in nonhuman primates] Psychologic Francaise. 1994;39:345–355. [Google Scholar]
- Andrew RJ. Evolution of facial expressions. Science. 1963a November 22;142:1034–1041. doi: 10.1126/science.142.3595.1034. [DOI] [PubMed] [Google Scholar]
- Andrew RJ. The origin and evolution of the calls and facial expressions of the primates. Behaviour. 1963b;20:1–109. [Google Scholar]
- Andrews MW, Rosenblum LA. Live social-video reward maintains joystick task performance in bonnet macaques. Perceptual and Motor Skills. 1993;77:755–763. doi: 10.2466/pms.1993.77.3.755. [DOI] [PubMed] [Google Scholar]
- Bard KA. Evolutionary foundations of intuitive parenting: A special case of maternal competence in chimpanzees. Early Development and Parenting. 1994;3:19–28. [Google Scholar]
- Bauer HR, Philip M. Facial and vocal individual recognition in the common chimpanzee. The Psychological Record. 1983;33:161–170. [Google Scholar]
- Boesch C, Boesch H. Mental map in wild chimpanzees: An analysis of hammer transports for nut cracking. Primates. 1984;25:160–170. [Google Scholar]
- Boysen ST, Berntson GG. Conspecific recognition in the chimpanzee (Pan troglodytes): Cardiac responses to significant others. Journal of Comparative Psychology. 1989;103:215–220. doi: 10.1037/0735-7036.103.3.215. [DOI] [PubMed] [Google Scholar]
- Brothers L. The neural basis of primate social communication. Motivation and Emotion. 1990;14:81–91. [Google Scholar]
- Burt DM, Perrett DI. Perception of age in adult Caucasian male faces: Computer graphic manipulation of shape and colour information. Proceedings of the Royal Society of London. 1995;259:137–143. doi: 10.1098/rspb.1995.0021. [DOI] [PubMed] [Google Scholar]
- Butler RA. The responsiveness of rhesus monkeys to motion pictures. Journal of Genetic Psychology. 1961;98:239–245. doi: 10.1080/00221325.1961.10534374. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. The recognition of social alliances by vervet monkeys. Animal Behaviour. 1986;34:1722–1731. [Google Scholar]
- Cheney DL, Seyfarth RM. How monkeys see the world. University of Chicago Press; Chicago: 1990. [Google Scholar]
- D'Amato MR, Salmon DP, Colombo M. Extent and limits of the matching concept in monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:35–51. doi: 10.1037//0097-7403.11.1.35. [DOI] [PubMed] [Google Scholar]
- Dasser V. A social concept in Java monkeys. Animal Behaviour. 1988;36:225–230. [Google Scholar]
- de Waal FBM. Chimpanzee politics. Harper & Row; Cambridge, MA: 1982. [Google Scholar]
- de Waal FBM, Aureli F. Consolation, reconciliation, and a possible cognitive difference between macaque and chimpanzee. In: Russon AE, Bard KA, Parker ST, editors. Reaching into thought: The minds of the great apes. Cambridge University Press; Cambridge, England: 1996. pp. 80–110. [Google Scholar]
- Dittrich W. Representation of faces in longtailed macaques (Macaca fascicularis) Ethology. 1990;85:265–278. [Google Scholar]
- Ekman E. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Ekman P, Oster H. Facial expressions of emotion. Annual Review of Psychology. 1979;30:527–554. [Google Scholar]
- Fouts RS, Chown B, Goodin L. Transfer of signed responses in American Sign Language from vocal English stimuli to physical object stimuli by a chimpanzee (Pan) Learning and Motivation. 1976;7:458–475. [Google Scholar]
- Gallup GGJ. Chimpanzees: Self-recognition. Science. 1970 January;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Human-like pattern of Wernicke's brain language area homolog. Science. 1998 January 9;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Goodall J. In the shadow of man. Houghton-Mifflin; Boston: 1971. [Google Scholar]
- Goodall J. The chimpanzees of Gombe. Harvard University Press; Cambridge, MA: 1986. [Google Scholar]
- Harcourt AH. Coalitions and alliances: Are primates more complex than nonprimates? In: Harcourt AH, de Waal FBM, editors. Coalitions and alliances in humans and other animals. Oxford University Press; Oxford, England: 1992. pp. 445–472. [Google Scholar]
- Hinde RA. Interactions, relationships and social structure. Man. 1976;11:1–17. [Google Scholar]
- Hinde RA, Rowell TE. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta) Proceedings of the Zoological Society of London. 1962;138:1–21. [Google Scholar]
- Hopkins WD, Marino L, Rilling J, MacGregor L. A comparative study of asymmetries in the planum temporale in primates as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Washburn DA, Hyatt CW. Video-task acquisition in rhesus monkeys (Macaca mulatta) and chimpanzees (Pan troglodytes): A comparative analysis. Primates. 1996;37:197–206. doi: 10.1007/BF02381407. [DOI] [PubMed] [Google Scholar]
- Huber E. The evolution of facial musculature and facial expressions. Johns Hopkins University Press; Baltimore: 1931. [Google Scholar]
- Humphrey NK, Keeble GR. The reaction of monkeys to “fearsome” pictures. Nature. 1974;251:500–502. doi: 10.1038/251500a0. [DOI] [PubMed] [Google Scholar]
- Itakura S. A chimpanzee with the ability to learn the use of personal pronouns. The Psychological Record. 1992;42:157–172. [Google Scholar]
- Izard CE. The face of emotion. Appleton-Century-Crofts; New York: 1971. [Google Scholar]
- Jolly A. Lemur social behavior and primate intelligence. Science. 1966;153:501–506. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- Kawai M. On the rank system in a natural group of Japanese monkeys. Primates. 1958;1:84–98. [Google Scholar]
- Keating CE, Keating EG. Monkeys and mug shots: Cues used by rhesus monkeys (Macaca mulatta) to recognize a human face. Journal of Comparative Psychology. 1993;107:131–139. doi: 10.1037/0735-7036.107.2.131. [DOI] [PubMed] [Google Scholar]
- Kummer H. Primate societies. Aldine; Chicago: 1971. [Google Scholar]
- Kyes RC, Candland DK. Baboon (Papio hamadryas) visual preferences for regions of the face. Journal of Comparative Psychology. 1987;101:345–348. [PubMed] [Google Scholar]
- Leavens D, Hopkins WD. Hand use and gestural communication in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Communication in monkeys and apes. In: DeVore I, editor. Primate behavior. Holt, Rinehart & Winston; New York: 1965. pp. 544–584. [Google Scholar]
- Marler P. Social organization, communication, and graded signals: The chimpanzee and the gorilla. In: Bateson PPG, Hinde RA, editors. Growing points in ethology. Cambridge University Press; London: 1976. pp. 239–279. [Google Scholar]
- Matsuzawa T. Color naming and classification in a chimpanzee (Pan troglodytes) Journal of Human Evolution. 1985;14:283–291. [Google Scholar]
- Matsuzawa T. Spontaneous pattern construction in a chimpanzee. In: Heltne PG, Marquardt LA, editors. Understanding chimpanzees. Harvard University Press; Cambridge, MA: 1989. pp. 252–265. [Google Scholar]
- McGrew WC. Chimpanzee material culture. Cambridge University Press; Cambridge, England: 1992. [Google Scholar]
- McKelvie SJ. The role of eyes and mouth in the memory of a face. American Journal of Psychology. 1976;89:311–323. [Google Scholar]
- Mendelson MJ, Haith MM, Goldman-Rakic PS. Face scanning and responsiveness to social cues in infant rhesus monkeys. Developmental Psychology. 1982;l8:222–228. [Google Scholar]
- Miller RE, Banks JH, Jr., Ogawa N. Role of facial expressions in “cooperative avoidance conditioning” in monkeys. Journal of Abnormal Social Psychology. 1963;67:24–30. [Google Scholar]
- Miller RE, Caul WE, Mirsky IA. Communication of affects between feral and socially isolated monkeys. Journal of Personality and Social Psychology. 1967;7:231–239. doi: 10.1037/h0025065. [DOI] [PubMed] [Google Scholar]
- Nahm FKD, Perret A, Amaral DG, Albright TD. How do monkeys look at faces? Journal of Cognitive Neuroscience. 1997;9:611–623. doi: 10.1162/jocn.1997.9.5.611. [DOI] [PubMed] [Google Scholar]
- Nishida T. The social structure of chimpanzees of the Mahale Mountains. In: Hamburg DA, McCown ER, editors. The great apes. Benjamin/ Cummings; Menlo Park, CA: 1979. pp. 73–121. [Google Scholar]
- Nissen HW, Blum JS, Blum RA. Analysis of matching behavior in chimpanzees. Journal of Comparative and Physiological Psychology. 1948;41:62–74. doi: 10.1037/h0061040. [DOI] [PubMed] [Google Scholar]
- Oden DL, Thompson RKR, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pantroglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. [PubMed] [Google Scholar]
- Oden DL, Thompson RKR, Premack D. Infant chimpanzees spontaneously perceive both concrete and abstract same/different relations. Child Development. 1990;61:621–631. [PubMed] [Google Scholar]
- Parr LA. [Comparison of performance on two NITS tasks] 1998 Unpublished raw data. [Google Scholar]
- Parr LA, de Waal FBM. Visual kin recognition in chimpanzees. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- Parr LA, Dove TA, Hopkins WD. Why faces may be special: Evidence of the inversion effect in chimpanzees (Pantroglodytes) Journal of Cognitive Neuroscience. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- Parr LA, Hopkins WD, de Waal FBM. The perception of facial expressions in chimpanzees (Pan troglodytes) Evolution of Communication. 1998;2:1–23. [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD. Is the inversion effect in rhesus monkeys (Macaca mulatta) face specific? Animal Cognition. 1999;2:123–129. [Google Scholar]
- Plimpton EH, Swartz KB, Rosenblum LA. Responses of juvenile bonnet macaques to social stimuli presented through color videotapes. Developmental Psychobiology. 1981;14:109–115. doi: 10.1002/dev.420140204. [DOI] [PubMed] [Google Scholar]
- Preuschoft S. “Laughter” and “smile” in Barbary macaques (Macaca sylvanus) Ethology. 1992;91:220–236. [Google Scholar]
- Preuschoft S, van Hooff JARAM. Homologizing primate facial displays: A critical review of methods. Folia Primatologica. 1995;65:121–137. doi: 10.1159/000156878. [DOI] [PubMed] [Google Scholar]
- Redican WK, Kellicutt MH, Mitchell G. Preferences for facial expressions in juvenile rhesus monkeys (Macaca mulatta) Developmental Psychology. 1971;5:539. [Google Scholar]
- Rosenfeld SA, Van Hoesen GW. Face recognition in the rhesus monkey. Neuropsychologia. 1979;17:503–509. doi: 10.1016/0028-3932(79)90057-5. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Monkeys reared in isolation with pictures as visual input: Evidence for an innate releasing mechanism. Science. 1966 December 16;154:1468–1473. doi: 10.1126/science.154.3755.1468. [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh S, McDonald K, Sevcik RA, Hopkins WD, Rubert E. Spontaneous symbol acquisition and communicative use by pygmy chimpanzees (Pan paniscus) Journal of Experimental Psychology. 1986;115:1–25. doi: 10.1037//0096-3445.115.3.211. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret J. Functional and anatomical decomposition of face processing: Evidence from prosopagnosia and PET study of normal subjects. Philosophical Transactions of the Royal Society of London B. 1992;335:55–62. doi: 10.1098/rstb.1992.0007. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Do monkeys understand their relations? In: Byrne RW, Whiten A, editors. Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford University Press; Oxford, England: 1988. pp. 69–84. [Google Scholar]
- Swartz KB, Rosenblum LA. Operant responding by bonnet macaques for color videotape recordings of social stimuli. Animal Learning and Behavior. 1980;8:311–321. [Google Scholar]
- Tomonaga M, Itakura S, Matsuzawa T. Superiority of conspecific faces and reduced inversion effect in face perception by a chimpanzee. Folia Primatologica. 1993;61:110–114. doi: 10.1159/000156737. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR, Damasio H. Intact recognition of facial expressions, gender, and age in patients with impaired recognition of face identity. Neurology. 1988;38:690–696. doi: 10.1212/wnl.38.5.690. [DOI] [PubMed] [Google Scholar]
- van Hooff JARAM. Sociality in primates, a compromise of ecological and social adaptation strategies. In: Tartabini A, Genta ML, editors. Perspectives in the study of primates. De Rose; Cosenza, Italy: 1988. pp. 11–22. [Google Scholar]
- Waser PM. Individual recognition, group cohesion, and intergroup spacing: Evidence from sound playback to forest monkeys. Behaviour. 1977;60:28–73. [Google Scholar]
- Washburn DA, Rumbaugh DM. Testing primates with a joystick-based automated apparatus: Lessons from the Language Research Center's computerized test system. Behavioral Resource Methods, Instruments and Computers. 1992;24:157–164. doi: 10.3758/bf03203490. [DOI] [PubMed] [Google Scholar]