Abstract
Magnetic resonance images (MRI) were collected in a sample of 28 apes, 16 Old World monkeys and 8 New World monkeys. The length of the sylvian fissure (SF) and the superior temporal sulcus (STS) was traced in each hemisphere from three regions of the cerebral cortex. These three regions were labeled according to their position on the sagittal plane as lateral, medial and insular. It was hypothesized that the length and asymmetry of these fissures would be dependent on the region of measurement and that a leftward asymmetry in the SF and STS would be more robust in the great ape sample than for the monkeys. The results indicated within the ape sample a population-level leftward asymmetry in the medial and insular regions of the SF. Within the Old and New World monkey samples, the SF was leftward in the medial region at the population level, but not at the insular region. Additionally, the Old World monkeys exhibited a population-level rightward lateral SF and a rightward lateral STS. No other families exhibited population-level asymmetries in the lateral region of the SF or in any region of the STS. These results are consistent with findings reported in apes and, to a lesser extent, monkeys. MRI has excellent potential for comparing neuroanatomy across taxonomic families that will help future investigations.
Keywords: Sylvian fissure, Magnetic resonance imaging, Non-human primates, Asymmetry
Introduction
Since the early reports of neuroanatomical asymmetries in humans by Dax, Broca and Wernicke, there has been an interest in knowing whether nonhuman species, notably primates, similarly exhibit morphological asymmetries in the brain as well as functional asymmetries [see Harris, 1993, for review]. From an evolutionary standpoint, the question of whether nonhuman species exhibit neuroanatomical asymmetries has been of interest because one of the major functional and neuroanatomical asymmetries in the human brain involves language processing. Specifically, most right-handed humans (and a statistical majority of left-handed individuals) are left hemisphere dominant for language [Rasmussen and Milner, 1977]. Moreover, gross anatomical as well as recent structural brain imaging studies have revealed left hemisphere morphological asymmetries in areas proposed to underlie language functions including the planum temporale and par triangularis [Geschwind and Levitsky, 1968; Foundas et al., 1995]. Of specific interest to this study are the reports of planum temporale asymmetries and associated asymmetries in temporal lobe sulci. Until recently, it was thought that the planum temporale could not be anatomically defined in nonhuman primates, but recent findings indicate that this region can be defined using landmarks from the human brain, at least in great apes [Gannon et al., 1998; Hopkins et al., 1998]. Prior to these studies, rather than assess planum temporale asymmetries directly, investigators estimated planum temporale asymmetries indirectly by measuring the length of the sylvian fissure.
In one of the earliest reports, Yeni-Komshian and Benson [1976] examined the length of the sylvian fissure (SF) in post-mortem brains of 25 humans, 25 chimpanzees and 25 rhesus monkeys. Methodologically, Yeni-Komshian and Benson used thread to line the SF from the anterior and posterior borders of the left and right hemispheres and measured the length after cutting the thread. The length of the SF was significantly longer in the left hemisphere compared to the right hemisphere in both the human and chimpanzee brains, whereas there was no significant asymmetry in the rhesus monkey brains. The findings of this left hemisphere asymmetry were significant because the perisylvian region reflects the planum temporale that corresponds roughly to Wernicke's area in human brains [Geschwind and Levitsky, 1968].
Since the report by Yeni-Komshian and Benson [1976] there have been a number of attempts to replicate these findings [Tan and Caliskan, 1987; Tan, 1992], particularly in nonhuman primates. To date, the findings from these studies have been equivocal and some of the results seem to be influenced by the methodology used to assess asymmetries. For example, in a series of studies, Falk and colleagues attempted to measure SF length from endocasts in various samples of Old World monkeys [Falk, 1986; Falk, et al., 1986, 1990]. In these studies, the braincase or inside of the skulls were filled with latex on which the sulci of the brain were imprinted. Upon removal of the latex, fissures on each hemisphere were identified and digitized for subsequent length analyses. Falk et al. [1986] initially reported evidence of a left hemisphere asymmetry in SF length from a sample of 10 rhesus monkey endocasts. However, in a follow-up study involving a larger sample of 335 rhesus monkeys, Falk et al. [1990] failed to find any population-level asymmetry in the length of the SF.
In contrast to the studies using endocasts, Heilbroner and Holloway [1988] examined SF length in a sample of Old and New World monkey cadaver brain specimens. The sample included 30 Macaca mulatta, 30 Macaca fasicularis, 27 Samiri oedipus, 31 Samiri sciureus, and 26 Callithrix jacchus. With respect to SF length, the anterior and posterior borders were anchored with pins and the length was measured from each hemisphere with flexible measuring tape. In contrast to the findings by Yeni-Komshian and Benson [1976], population-level left hemisphere asymmetries were found for Macaca mulatta, Macaca fasicularis, Callithrix jacchus and Samiri oedipus. Significant population-level asymmetries were not found for Samiri sciureus. Heilbroner and Holloway [1988] also photographed each hemisphere in the Macaca mulatta brain specimen sample and traced the SF length for each hemisphere. These data were compared to the measurements taken directly from the cadaver specimens. Although population-level asymmetries were present in cadaver specimens, no effect was found in the measurements taken from the photographs. Heilbroner and Holloway [1988] argue that by using photographs, aspects of asymmetry in the length of the SF are lost because the two-dimensional image did not capture the shape of the brain as it relates to the overall length of various fissures.
The use of endocasts compared to cadaver specimens has resulted in conflicting findings with respect to asymmetries in SF length of monkeys. Although each method has its own merits, one problem with both of these approaches is that neither accounts for the possibility that part of the SF can be buried in the cortex and therefore is not projected onto the surface of the brain. The purpose of this study was to compare SF length between the hemispheres in a sample of great apes, Old World monkeys and New World monkeys using magnetic resonance imaging (MRI). One advantage of MRI is that it allows for measurement of the SF (and other fissures) at various levels of the cortex and therefore allows for an analysis of SF variation on the lateral compared to medial sagittal views of the brain. If SF asymmetries are uniform and consistent among species, then population-level asymmetries should be evident in all taxonomic families. In contrast, if there is phylogenetic discontinuity in SF asymmetries, such as those reported by Yeni-Komshian and Benson [1976], then great apes should show a left hemisphere asymmetry whereas Old and New World monkeys should not.
Materials and Methods
Subjects
Magnetic resonance images (MRI) were collected in a sample of 28 great apes, which included 18 chimpanzees (Pan troglodytes), 4 bonobos (Pan paniscus), 4 orangutans (Pongo pygmaeus) and 2 gorillas (Gorilla gorilla); 16 Old World monkeys, which included 2 baboons (Papio papio), 4 sooty mangabeys (Cercocebus torquatus atys), and 10 rhesus (Maccaca mulatta); and 8 New World monkeys, which included 4 capuchins (Cebus apella) and 4 squirrel monkeys (Saimiri sciureus). The distribution of sex within family was 14 females and 14 males for the apes (Pongidae), 3 females and 13 males for the Old World monkeys (Cercopithecidae) and 3 females and 5 males for the New World monkeys (Cebidae). Seven of the 28 chimpanzee brains were scanned postmortem (the remaining subjects were alive and healthy at the time of data collection). These cadaver brains were stored in tap water and 10% formaldehyde for varying intervals of 1 week to 5 years. Only three cadaver brains remain in the authors' possession after scanning, therefore surface measurements were not completed.
Procedure
The subjects were first immobilized by ketamine injection (l0 mg/kg) and subsequently anesthetized with propofol (40–60 mg/kg/h) following standard procedures at Yerkes Regional Primate Research Center (YRPRC). Subjects were transported by van to the MRI facility at Emory University Hospital. The subjects remained anesthetized for the duration of the scans as well as the time needed to travel between YRPRC and Emory Hospital (total time ∼2 h). At the MRI facility, the apes were placed in the scanner chamber with their head fitted inside the head coil and monkeys were placed with their head fitted inside the knee coil. The cadaver brains were placed inside the knee coil dorsal side up and the front of the brain facing outside the scanner. Scan duration, a function of brain size, was approximately 40 to 80 minutes. This project involved using two MRI machines (Phillips, Model 51), each with 1.5-Tesla super conducting magnets. For all subjects, T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 0.6 mm, number of signals averaged = 8 and a 256 × 256 matrix). These scan parameters were used based on preliminary studies and provided excellent resolution of the brain areas of interest to this study. After completing MRI procedures, the subjects were returned to YRPRC and temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage. Cadaver brains were placed in a knee coil with the dorsal view up. The archived data were stored on optical diskettes and transported to a Sun Sparc work station where multiplanar reformatting software (Easy Vision) was used to align the scans in the coronal, sagittal and transverse planes. The images were cut into l-mm sagittal slices for post-image processing. Post-image analyses were performed using the PC version of NIH Image software (Scion Corporation).
Fissure Measurements
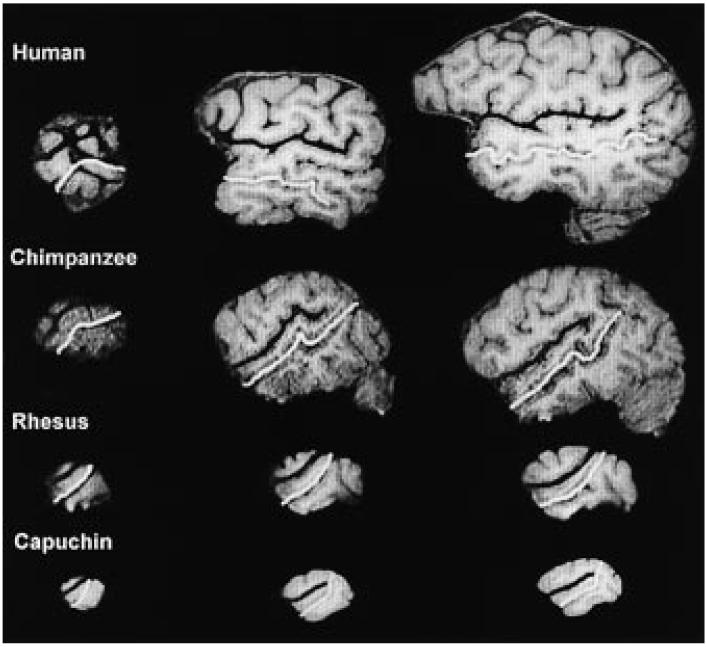
A mouse-driven computer-guided cursor controlling a free-hand line tool with no anchors was used to measure the length of the sylvian fissure (SF) and superior temporal sulcus (STS) in the left and right hemispheres. The SF and STS were traced from the anterior to the posterior temporal region on three sagittal scans of each hemisphere (in tenths of mm; see figure 1). The reformatted sagittal slices from which the measurements were taken were selected by following a standard procedure for all subjects. Specifically, the number of 1 mm slices was counted from the lateral surface of the hemisphere to the slice on which the insula could be identified. The first measure of length was taken on the lateral surface, which was defined as the first sagittal scan that both the SF and STS appeared (LSF; LSTS). Continuing medially, a second measure of length was taken from the scan immediately before the opening of the insula, which appeared as a split in the SF (ISF; ISTS). A third tracing was taken from the scan that was numerically halfway between the scans of the lateral and insular measures (MSF; MSTS). If an even number of scans fell between the lateral and insular regions, the length from the two most medial were averaged. Only 6 of the 8 New World monkeys had at least one slice between the lateral and insular regions, therefore two Samiri oedipus did not have a medial measure for the SF and STS. In this sample, bifurcation of the SF and STS at the posterior end occurred inconsistently, therefore the tracing terminated before any ascending or descending ramus.
Fig. 1.
MRI images from the sagittal plane of a human, common chimpanzee, rhesus macaque and capuchin monkey demonstrate the scans that were identified as the lateral, insular and medial regions for measurements of the SF (black tracing) and STS (white tracing). The human data are only presented in the figure for the purposes of comparison and were not included as part of the data analysis.
Data Analysis
For each sulcus (SF, STS) and region (lateral, medial, insular), an asymmetry quotient (AQ) was calculated using the formula [(R–L)/((R+L) * 0.5)]. The sign of the resulting value indicated the direction of asymmetry (positive value = right hemisphere bias; negative value = left hemisphere bias). The inter-rater reliability of two investigators was examined for the length of the SF of 10 randomly selected subjects. The inter-rater correlation coefficient for the left and right hemisphere of the SF was 0.948 and 0.910, respectively.
Results
To investigate any relationships between the AQ for the different regions of the SF and STS, a Pearson product-moment correlation was performed. For the SF, only the medial and insular AQ values for the great ape sample were significantly correlated (r(28) = 0.81, p < 0.01). The AQs of the lateral and insular (r(28) = 0.49, p < 0.01) and medial and insular (r(28) = 0.38, p < 0.05) regions of the STS in the ape sample were also significantly related. There were no significant relationships between the AQ values of any region of the SF or STS in the sample of monkeys. To assess directional asymmetry of the SF and STS, one-sample t tests were performed on the measures from the lateral, medial and insular regions between each taxonomic family. The mean AQ scores for each SF and STS section across taxonomic family can be found in table 1. Within the great ape sample, population-level left hemisphere biases were found for the MSF (t(27) = −9.63, p < 0.01) and ISF (t(27) = −3.65, p < 0.01) regions. Within the sample of Old World monkeys, a significant rightward population-level asymmetry was found for the LSF (t(15) = 3.73, p < 0.01) and a leftward population-level asymmetry for the MSF (t(15) = −9.10, p < 0.01). A leftward population-level asymmetry was also seen in the sample of New World monkeys for the MSF (t(7) = −5.99, p < 0.01). In terms of the STS, rightward population-level asymmetries were found only for the LSTS of the Old World monkeys (t(15) = 3.18, p < 0.01), which is consistent with results found for the lateral region of the SF. No significant asymmetries were evident for the STS in any other region or for any other family.
Table 1.
The mean asymmetry quotient (AQ) and standard error (s.e.) is shown for each section of the sylvian fissure (SF) and superior temporal sulcus (STS) across taxonomic family
| Sylvian fissure |
Superior temporal sulcus |
|||||
|---|---|---|---|---|---|---|
| LSF (s.e.) | MSF (s.e.) | ISF (s.e.) | LSTS (s.e.) | MSTS (s.e.) | ISTS (s.e.) | |
| Great apes | −0.011 (0.042) | −0.324 (0.034) | −0.082 (0.023) | −0.001 (0.049) | −0.006 (0.034) | −0.013 (0.022) |
| Old World monkeys | 0.218 (0.058) | −0.370 (0.041) | −0.003 (0.021) | 0.133 (0.042) | 0.036 (0.024) | 0.017 (0.020) |
| New World monkeys | −0.000 (0.107) | −0.268 (0.045) | −0.007 (0.023) | 0.043 (0.118) | 0.037 (0.055) | −0.009 (0.026) |
The mean AQ values that are boldfaced indicate significant asymmetries (p < 0.05).
In addition to the AQ data, subjects were classified as exhibiting either a left- or right-hemisphere bias based on the magnitude and sign of their AQ value [Hopkins et al., 1998]. Subjects with negative values lower than −0.025 were classified as having a left hemisphere asymmetry, whereas subjects with a positive AQ value greater than 0.025 were classified as having a right hemisphere asymmetry. Subjects with an AQ value between −0.025 and 0.025 were classified as having no bias. Whether the number of individuals exhibiting a left or right hemisphere asymmetry differed from chance within each region was assessed using a binomial z score. The number of individuals that exhibited left, right or no asymmetry for the SF and STS is shown in table 2. As with the AQ results, significant left hemisphere asymmetries were found for the medial SF for the great apes, Old World monkeys and New World monkeys. In addition, a significant proportion of the great ape sample exhibited a left hemisphere asymmetry for the insular measure of the SF. The only other significant finding was a right hemisphere asymmetry for the lateral SF measure in the Old World monkeys.
Table 2.
The frequency of leftward, rightward, or absence of asymmetry according to the asymmetry quotient values (AQ) for each region of the sylvian fissure (SF) and superior temporal sulcus (STS) across taxonomic family. The binomial z score is also presented based on the probability of a leftward asymmetry
| Lateral |
Medial |
Insular |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L > R | R > L | R = L | z | L > R | R > L | R = L | z | L > R | R > L | R = L | z | |
| SF | ||||||||||||
| Great apes (n = 28) | 11 | 16 | 1 | −1.13 | 28 | 0 | 0 | 5.29 | 22 | 4 | 2 | 3.02 |
| Old World monkeys (n = 16) | 4 | 12 | 0 | −2.00 | 16 | 0 | 0 | 4.00 | 9 | 5 | 2 | 0.50 |
| New World monkeys* (n = 8) | 4 | 4 | 0 | 0.00 | 6 | 0 | 0 | 2.45 | 4 | 4 | 0 | 0.00 |
| STS | ||||||||||||
| Great apes (n = 28) | 12 | 13 | 3 | −0.76 | 11 | 12 | 5 | −1.13 | 12 | 12 | 4 | −0.76 |
| Old World monkeys (n = 16) | 2 | 13 | 1 | −3.00 | 1 | 9 | 6 | −3.50 | 4 | 8 | 4 | −2.00 |
| New World monkeys* (n = 8) | 4 | 4 | 0 | 0.00 | 2 | 3 | 1 | −0.82 | 3 | 3 | 2 | −0.71 |
Two New World monkeys had only one scan before the opening of the insula, therefore only a lateral and insular measure of the SF and STS were possible. Numbers highlighted in bold indicate significant z-scores.
The results from this study reveal that the region or depth of the cortex from which the SF or STS is measured significantly predicts the presence and direction of hemispheric asymmetry. In the great ape sample, the SF was significantly longer on the left hemisphere in both the medial and insular regions, but there were no asymmetries at the lateral surface. A leftward asymmetry in the medial region of the SF was also present in both the New and Old World monkey sample. In addition, the Old World monkeys not only exhibited a rightward asymmetry of the LSF, but also a rightward asymmetry in the LSTS. As for the STS in the apes, there were no significant asymmetries despite strong leftward asymmetries seen at the medial and insular level of the SF.
Discussion
The findings reported in this study support the previous results by Yeni-Komshian and Benson [1976], for their chimpanzee sample, but are inconsistent with their findings in Old World monkeys. With respect to the great ape data, this is the first study to report findings consistent with those of Yeni-Komshian and Benson [1976], which suggests that SF asymmetries in great apes, and particularly chimpanzees, are robust even when using different methods of assessment. Studies that investigate the SF of New and Old World monkeys often contradict each other, which is possibly due to methodological inconsistencies. There is, however, one consistency across all studies of monkeys and apes regardless of the use of endocasts or brain specimens, and that is the measurement of the sylvian fissure on the lateral surface of the brain. It is hypothesized that the length of the SF should change as it folds within the cortex, therefore, it would be expected that there would be some variability in the length of the SF depending on the region of measurement and possibly the method used to measure the sulcus. Surprisingly, the ape sample in this study was not significantly lateralized on the surface of the brain, which is the region of the SF reported to be lateralized by YeniKomshian and Benson [1976] . On the other hand, we found that both the SF and STS of the Old World monkey sample were significantly lateralized on the lateral surface. These findings from the monkey sample are consistent with studies by Falk [1978] and Falk et al. [1990], although the rightward asymmetry did not reach significance in the latter. These findings are, however, inconsistent with several other studies of SF length from endocasts [Falk et al., 1986; Heilbroner and Holloway, 1988] and cadaver specimens [YeniKomshian and Benson, 1976].
This variability in sulcal length at different depths of the cortex for both the monkey and ape sample is of particular interest from a comparative neuropsychological perspective. The increase in laterality as one ascends the order primate, according to the greater leftward asymmetry in the SF, might be a reflection of increased functional capabilities for behavior organized in the temporal region of the brain. This notion of laterality is based on the assumption that a longer SF infers a larger three-dimensional area for the corresponding cortex. Leftward asymmetry of the SF in the New and Old World monkeys is apparent at the medial region only, although within the ape sample, a significant asymmetry is apparent much deeper into the temporal cortex, indicating a larger area of lateralization. Moreover, the symmetry found for the STS and the leftward asymmetry reported for the SF in the ape sample further supports the notion that the cortex surrounding the SF, only a few centimeters dorsal to the STS, is significantly lateralized. The rightward lateral and leftward medial asymmetries in the Old World monkeys might be a reflection of a phylogenetic increase in leftward laterality of the temporal region as the New World monkeys exhibited no asymmetry in the lateral region and a leftward medial asymmetry.
Although the effects are relatively large, the sample sizes for the monkey data are small, particularly with respect to representation of different species within each family. This presents the need for some caution in generalizing the results across species. An additional limitation to this study, also regarding the small sample size for both apes and monkeys, is the inability to control for the subject's age and the absence of statistical power to test for sex differences. Age and sex could account for some variability in strength and region of asymmetry, particularly in the Old World monkey sample (3 females and 13 males).
Finally, this study focused on asymmetries in the comparative neuroanatomy of the SF and STS and does not address the issue of the function of these asymmetries in the context of primate evolution. Asymmetries in the SF are thought to reflect an expansion of the posterior temporal lobe, and particularly the region that corresponds roughly to Wernicke's area in the human brain [see Bradshaw and Rogers, 1993]. One interpretation of the presence of SF asymmetries in all three primate families is that the homolog to speech and language processing has its origin early in primate evolution (and perhaps in mammalian evolution). The primary data in support of this conclusion are the behavioral evidence of a left hemisphere advantage in the discrimination of species-specific vocalizations in Japanese macaques [Petersen et al., 1978] and the evidence that lesions in the posterior portion of the left but not right hemisphere disrupt this discrimination [Heffner and Heffner, 1984]. An alternative interpretation is that the SF asymmetries reflect a general advantage of the left hemisphere in processing sequential auditory information and is not specific to species-specific calls. For example, some studies have reported a left hemisphere advantage for discriminating sequential, auditory information in monkeys [baboons; Pohl, 1983, 1984] and lesions to the posterior portion of the left hemisphere do disrupt discrimination performance [Dewson, 1977]. Finally, it is possible that the morphological asymmetries in the perisylvian region support different functions in different species or alternatively are not involved in auditory perception but rather associates with other functional asymmetries, such as hand preference [see Hopkins and Carriba, in press]. A preliminary analysis of the association between the SF asymmetries reported here and measure of hand preference in the chimpanzees were not significant (r values were −310, 0.416, and 0.268) but perhaps with a larger sample these associations would reach conventional levels of statistical significance. In short, in the absence of studies that specifically address the structure and function of the perisylvian fissure area in the same sample of subjects, it would be premature to state any definitive conclusions about the function of the SF asymmetry in different primates.
In conclusion, this study is the first to use MRI to measure sylvian fissure length in nonhuman primates. In addition, this is the first study that has used the variability in the length of the SF as it buries itself within the temporal cortex as a means of assessing SF asymmetries. The variability in the length of the SF might contribute to the inconsistent findings reported from endocasts and cadaver specimens of non-human primates and we suggest that MRI is particularly useful for avoiding this potential problem in comparative neuroanatomy studies. With the use of MRI for comparative neuroanatomy, the field of neuropsychology might be able to resolve methodological inconsistencies that have constrained our ability to understand the phylogeny of hemispheric asymmetries in primates.
Acknowledgements
This work was supported in part by NIH grants RR-00165, NS-29574, NS-36605 and HD38051. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care and APA guidelines for the ethical treatment of animals were adhered to during all aspects of this study. Drs. Jim Rilling and Tom Insel assisted in the collection of a significant portion of the MRI scans. Special thanks are also directed to Dr. Brent Swenson and the rest of the veterinary staff for assisting in the care of the animals during scanning.
References
- Bradshaw JL, Rogers L, editors. The Evolution of Lateral Asymmetries, Language, Tool-Use and Intellect. Academic Press; San Diego, Calif.: 1993. [Google Scholar]
- Dewson JH. Preliminary evidence of hemispheric asymmetry of auditory function in monkeys. In: Harnad S, Doty RW, Goldstein L, Jaynes J, Krauthamer G, editors. Lateralization in the Nervous System. Academic Press; New York: 1977. pp. 63–74. [Google Scholar]
- Falk D. Cerebral asymmetry in Old World monkeys. Acta Anat. 1978;101:334–339. [PubMed] [Google Scholar]
- Falk D. Endocranial casts and their significance for primate brain evolution. In: Swindler R, Erwin J, editors. Comparative Primate Biology. Vol. 1. Systematics, Evolution and Anatomy. Alan R. Liss; New York, N.Y.: 1986. pp. 477–490. [Google Scholar]
- Falk D, Cheverud J, Vannier MW, Conroy GC. Advanced computer graphics technology reveals cortical asymmetry in endo-casts of rhesus monkeys. Folia Primatol. 1986;46:98–103. doi: 10.1159/000156242. [DOI] [PubMed] [Google Scholar]
- Falk D, Hildebolt C, Cheverud J, Vannier M, Helmkamp C, Konigsberg L. Cortical asymmetries in frontal lobes of Rhesus monkeys (Macaca mulatta) Brain Res. 1990;512:40–45. doi: 10.1016/0006-8993(90)91167-f. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphologic cerebral asymmetries and handedness: the pars triangularis and planum temporale. Arch. Neurol. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's brain language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in the temporal speech region. Science. 1968;151:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Harris LJ. Handedness in apes and monkeys: some views form the past. In: Ward JP, Hopkins WD, editors. Primate Laterality: Current Behavioral Evidence of Primate Asymmetries. Springer Verlag; New York: 1993. pp. 1–44. [Google Scholar]
- Heffner HE, Heffner RS. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science. 1984;226:75–76. doi: 10.1126/science.6474192. [DOI] [PubMed] [Google Scholar]
- Heilbroner PL, Holloway RL. Anatomical brain asymmetries in New World and Old World monkeys: stages of temporal lobe development in primate evolution. Am. J. Phys. Anthropol. 1988;76:39–48. doi: 10.1002/ajpa.1330760105. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Carriba SF. Laterality in communicative behaviors in nonhuman primates: a critical analysis. In: Rogers L, Andrews R, editors. Comparative Vertebrate Lateralization. Oxford University Press; Oxford, UK: in press. [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Petersen MR, Beecher MD, Zoloth SR, Moody DB, Stebbins WC. Neural lateralization of species-specific vocalizations in Japanese macaques (Macaca fuscata) Science. 1978;202:324–327. doi: 10.1126/science.99817. [DOI] [PubMed] [Google Scholar]
- Pohl P. Central auditory processing: ear advantages for acoustic stimuli in baboons. Brain Lang. 1983;20:44–53. doi: 10.1016/0093-934x(83)90031-7. [DOI] [PubMed] [Google Scholar]
- Pohl P. Ear advantages for temporal resolution in baboons. Brain Cogn. 1984;3:438–444. doi: 10.1016/0278-2626(84)90033-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann. N.Y. Acad. Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Tan U, Caliskan S. Asymmetries in the cerebral dimensions and fissures of the dog. Int. J. Neurosci. 1987;32:943–952. doi: 10.3109/00207458709043351. [DOI] [PubMed] [Google Scholar]
- Tan U. Similarities between the sylvian fissure asymmetries in cat brain and planum temporale asymmetries in human brain. Int. J. Neurosci. 1992;66:163–175. doi: 10.3109/00207459209003303. [DOI] [PubMed] [Google Scholar]
- Yeni-Komshian GH, Benson DA. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees, and rhesus monkeys. Science. 1976;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]