Abstract
Neuroanatomical asymmetries have been identified in chimpanzee frontal and temporal lobes including regions believed to be homologous to human Broca's and Wernicke's areas. This study examined whether or not neuroanatomical asymmetries in chimpanzees are associated with hand use during gestural communication. Analyses revealed that those chimpanzees that reliably employ their right hand for manual gestures have larger inferior frontal gyri in the left hemisphere than those apes that do not show consistent hand use for gestures. These findings are the first to provide a direct link between neuroanatomical asymmetries and the production of lateralized communicative behavior in non-human primates.
Keywords: chimpanzee, gesture, inferior frontal gyrus, language evolution, neuroanatomical asymmetry
Introduction
Language is processed asymmetrically in the human brain, with the left hemisphere dominant compared with the right. This lateralization is functionally significant [1], modality independent [2,3], and is associated not merely with the perception or production of utterances but with their meaning [4,5]. It has been suggested that this hemispheric specialization for language evolved from a lateralized manual communication system that arose in a common human and chimpanzee ancestor [6]. Consistent with this theory are data that indicate that chimpanzees intentionally and referentially communicate via manual gestures [7,8], and, like humans [9], preferentially use their right hand for communicative gestures [10,11]. Human-like left hemisphere neuroanatomical asymmetries also have been identified in both the posterior temporal lobe and inferior frontal regions of the chimpanzee brain [12,13], regions considered homologous to Broca's and Wernicke's areas, respectively.
Previous work has demonstrated that handedness for noncommunicative manual actions in chimpanzees are associated with asymmetries of the primary motor cortex, but not with the homologous language regions [14]. The purpose of this study was to assess whether or not morphological differences between hemispheres in the chimpanzee inferior frontal gyrus (IFG) were evident in captive chimpanzees based on the hand they most frequently used for manual communicative gestures. To accomplish this aim, anatomical asymmetries were compared between chimpanzees classified as right-handed and non-right-handed for manual gestures. For comparison, the same chimpanzees were then re-classified for hand use during a noncommunicative motor action, and their anatomical asymmetries were compared. This was done to isolate the potential differential expression of motor actions for communicative and noncommunicative behaviors on brain asymmetries. The hypothesis was that hand use associated with communicative behavior would be associated with asymmetries in the IFG while hand use for noncommunicative actions would not.
Methods
Subjects
Fifty-six chimpanzees (28 males, 28 females) housed at the Yerkes National Primate Research Center (YNPRC) were included in this study. The chimpanzees were selected from a larger cohort of animals for which behavioral data on hand use [10,11,15] and magnetic resonance images (MRIs) had been collected previously.
Behavioral measures of hand use
Two behavioral measures of hand use were assessed in the chimpanzees: (1) simple reaching and (2) communicative manual gestures. These two measures were selected for comparison because they both require simple, unimanual, ballistic actions of the hand, but differ in that one is used in a communicative context and one is not. The exact procedures used to assess handedness for these two measures have been described in detail elsewhere [11,14,16,17]. In addition, a description of each task is included below. Both measures show consistent test–retest reliability and reveal population-level right-handedness, although the magnitude of rightward asymmetry is significantly higher for manual gestures than for simple reaching [11].
Manual gesturing (gesture)
At the onset of each trial, an experimenter would approach the chimpanzee's home cage and center themselves in front of the chimpanzee at a distance of approximately 1.0–1.5 m. If the chimpanzee was not already positioned in front of the experimenter at the onset of the trial, the chimpanzee would immediately move towards the front of the cage when the experimenter arrived with the food. The experimenter then called the chimpanzee's name and offered a piece of food until the chimpanzee produced a manual gesture. Only responses in which the chimpanzees unimanually extended the digit(s) through the cage mesh to request the food were considered a response. Other possible manual responses such as cage banging or clapping were not counted as a gesture. Two-handed gestures, although rare, were not scored as were gestures that were produced by the chimpanzee prior to the experimenter arriving in front of the chimpanzee's home cage. Chimpanzees were tested over a 15-day period, and a minimum of 30 responses was collected from each chimpanzee. The number of trials administered on a given day varied with the chimpanzee's motivation and availability for testing. Chimpanzees were tested in both the indoor and outdoor sections of their home enclosures, and none of the chimpanzees were separated from their groups or cage mates during testing. Hand use was recorded as right or left for each response (Fig. 1a).
Fig.1.
Asymmetry of brain regions as a function of handedness for communicative manual gestures and simple reaching in chimpanzees. (a) Chimpanzee gesture and reach. (b) Magnetic resonance image of chimpanzee inferior frontal gyrus (IFG) and motor hand area (KNOB). (c) Mean asymmetry quotients and standard errors for the IFG and KNOB for those chimpanzees that are right-handed and non-right-handed for communicative manual gestures and for simple reaching.
Simple reaching (reach)
For each trial, an experimenter would throw a small piece of food into the chimpanzee's home cage. The food was thrown so as to land at a minimum distance of 3 m from the chimpanzee. This required the chimpanzee to move to the location where the food had landed, reach to pick up the food, and then bring the food to their mouth. The chimpanzee was required to reposition themselves between each trial and retrieve the food item before another was thrown into the cage (Fig. 1a). Approximately 15–30 s separated each trial. Only one reaching response was recorded for each trial, and a minimum of 50 responses was obtained from each chimpanzee. The experimenter recorded hand use as right or left for each reaching response.
For both the behavioral measures, the chimpanzees were divided into two groups based on the frequency of left and right hand use. To accomplish this, a z-score was calculated for each o chimpanzee, and those individuals with z-scores ≥1.96 (P<0.05) were classified as right-handed (RH) for that behavioral measure. Individuals with z-scores ≤1.95 were classified as non-right-handed (NRH). We classified chimpanzees as RH and NRH rather than the more traditional right-handed and left-handed approach because there were very few left-handed individuals in the sample (n=6) for manual gestures. Combining the left-handed and the no-preference chimpanzees into one NRH category resulted in more comparable sample sizes of RH and NRH chimpanzees.
Magnetic resonance image acquisition
MRIs were collected from those chimpanzees for which behavioral data were available. The brain regions of interest included the IFG, planum temporale (PT), and motor hand area (KNOB). These regions were selected because morphological asymmetries have been identified previously in all three areas in chimpanzees [12-14].
Magnetic resonance image collection procedures
MRIs of the brain were collected in a sample of 56 chimpanzees (Pan troglodytes) including 28 males and 28 females. All the chimpanzees were housed at the YNPRC, and were alive and healthy at the time of behavioral data collection. MRI scans, however, were performed on nine of the chimpanzees postmortem. The cadaver specimens were stored in 10% buffered formalin solution for intervals ranging from 1 week to 2 years. For in-vivo scans, chimpanzees were first immobilized by ketamine injection (10 mg/kg) and subsequently anesthetized with propofol (40–60 mg/kg/h) following standard procedures at the YNPRC. Chimpanzees were then transported to the MRI facility. The chimpanzees remained anesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time ∼2 h). Chimpanzees were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 80 min as a function of brain size. After completing MRI procedures, the chimpanzees were returned to the YNPRC and temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage.
The majority (n=52) of the chimpanzees were scanned using one of two 1.5 T scanners (Phillips, Model 51, Philips Medical Systems, N.A., Bothell, Washington, USA). The remaining four chimpanzees were scanned using a 3.0 T scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA) at the YNPRC. The scanning methods have been described in detail elsewhere [14]. For all chimpanzees scanned in vivo using the 1.5 T machine, T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition=19.0 ms, echo time=8.5 ms, number of signals averaged=8, and a 256 × 256 matrix). For the nine postmortem scans using the 1.5 T scanner, T2-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition=3000 ms, echo time=40.0 ms, number of signals averaged=4, and a 256 × 256 matrix). These scan parameters were developed in previous studies [18], and provided excellent resolution of the brain areas of interest to this study. Four of the chimpanzees were scanned using a 3.0 T scanner (Siemens Trio). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition=2300 ms, echo time=4.4 ms, number of signals averaged=3, matrix size=320 × 320). The archived MRI data were transferred to a PC running Analyze 6.0 (Mayo Clinic, Mayo Foundation, Rochester, Minnesota, USA) software for postimage processing.
Quantification of the regions of interest
Quantification of the IFG, PT, and KNOB are described in detail elsewhere [12,14,18]. In addition, a description of the methods used for each region is included below. For the IFG, the MRI scans were aligned in the sagittal plane and cut into 1 mm slices. Consecutive parasagittal slices were traced from the lateral surface of the IFG to the most medial slice immediately preceding the insula. We defined the IFG as the portion of cortical surface bounded by the fronto-orbital and the precentral-inferior (PCI) sulci (see Fig. 1b). Both fronto-orbital and PCI could be clearly seen in parasagittal (1-mm thick) MRI slices.
For the PT, the MRI scans were aligned in the coronal planes and cut into 1 mm slices. The anterior border of the PT was defined by the most frontal slice in which Heschl's gyrus could be visualized. The posterior border was defined as the most caudal slice containing the sylvian fissure. The width of the PT was measured on each slice and then summed across all slices for each hemisphere.
The KNOB was localized in serial 1 mm slices in the axial plane. The dorsal and ventral edges of the KNOB served as the markers for defining the boundaries of the area (Fig. 1b). For each slice and hemisphere, an area measurement of the region was calculated through the use of a mouse-driven pointer that traced the region of interest. The total areas from all slices in which the KNOB was present were summed and used to derive a volume of the KNOB for each hemisphere.
Data analysis
For each region of interest, an asymmetry quotient (AQ) was obtained by using the following formula: AQ=[R−L/(R + L)(0.5)], where R and L represent the size of the right and left regions, respectively. Prior to statistical analysis, SPSS 13.0 (SPSS, Inc., Chicago, Illinois, USA) was used to construct box plots and to identify extreme AQ values for each brain region of interest. Box plots include the interquartile range, and the 5 and 95% confidence intervals as indicated by error bars located outside of the box. AQ values that were outside the confidence interval were identified as outliers and were removed from further analysis.
Results
On the basis of their z-scores for handedness, 33 of the 56 chimpanzees were classified as RH for communicative gestures, whereas 23 showed no preference or a left-hand preference (NRH). Similarly, 24 chimpanzees were classified as RH and 32 as NRH for simple reaching.
For handedness associated with manual gestures, univariate analysis of variance revealed a significant difference in the AQ scores for the IFG but not the KNOB and PT between RH and NRH chimpanzees F(1)=7.20, P=0.01 (Fig. 1c). In contrast, for simple reaching, there was a significant difference in the AQ scores between RH and NRH chimpanzees for the KNOB F(1)=7.27, P=0.01 (Fig. 1c) but not the PT or IFG. The mean AQ scores for the IFG and for the KNOB for both handedness measures are shown in Fig. 1c. In addition, Fig. 2 depicts the individual AQ scores for the IFG for chimpanzees that are RH and NRH for communicative manual gestures.
Fig. 2.
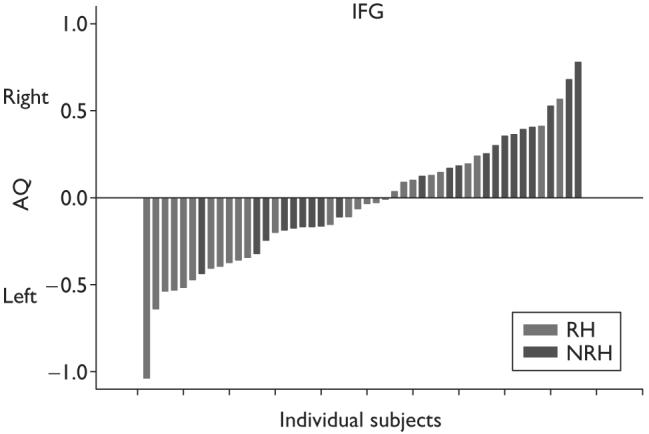
Individual asymmetry quotient (AQ) scores for the inferior frontal gyrus (IFG) for those chimpanzees that are right-handed and non-right-handed for communicative manual gestures.
Discussion
The results of this study indicate that chimpanzees that show a significant right-hand preference for manual gestures have a larger left compared with right IFG, whereas no differences are observed for the PT or KNOB. In contrast, hand preferences for simple reaching are associated with asymmetries in the KNOB but not in the IFG or PT, a finding that confirms earlier reports [14]. Thus, asymmetries in the IFG were specifically associated with communicative manual actions, whereas structural lateralization of the KNOB was related to a similar behavior lacking the communicative component. These data represent the first evidence associating chimpanzees' communicative behavior with neuroanatomical asymmetries. These findings are also consistent with reports from human subjects that indicate that the left IFG is activated during the pronunciation of deictic sentences [19].
Some have suggested that there is too much variability in IFG sulci, particularly in the PCI, which comprise the landmarks of the frontal operculum in great apes, to obtain a reliable assessment of asymmetry in this region [20]. While variability is observed in the sulci comprising the frontal operculum of apes, we believe that consistent criteria can be applied to this region to obtain reliable measurements. Specifically, the majority of cases of PCI bifurcation we have observed in our sample of chimpanzee brains follows a typical pattern in which the posterior ramus of the bifurcation (a) terminated at a more dorsal level than the anterior one, and (b) tended to run more horizontally than the anterior ramus, which in turn ran more vertically and parallel to the central sulcus. Notably, Sherwood and colleagues [20] gave a similar account for this usual pattern of PCI bifurcation in their cadaver specimens. Thus, in our experience, most instances of PCI bifurcation do not present any serious ambiguity as to determining the posterior boundary of the pars opercularis. As (a) PCI is usually seen as running vertically and roughly parallel to the central sulcus in the common sulcal pattern of the IFG of great apes and (b) in many instances, it is likely that the posterior ramus of a PCI bifurcation is, in fact, the subcentral anterior sulcus joining PCI (as pointed out by Sherwood et al. [20], as well), one can consistently adopt the criterion of choosing the anterior ramus of a PCI bifurcation as the posterior boundary for the pars opercularis. In point of fact, this criterion was consistently employed in the Cantalupo and Hopkins [12] report of a population-level leftward asymmetry of the pars opercularis in great apes. Further, a recent study using novel asymmetry measures based on gyrification estimates of the IFG showed a clear trend for a population-level leftward asymmetry for this brain region [21], in congruence with prior findings [12]. Thus, we argue that, far from being an insurmountable problem, the morphological variability of the frontal operculum in great apes can be usefully exploited as a valuable source of information in assessing the presence of macrostructural hemispheric asymmetries in these species.
Sherwood et al. [20] also questioned whether the cytoarchitectonic map of the IFG mapped entirely as Brodmann's area 44 cells or whether other cells were found in this region, notably Brodmann's area 45 and 46 cells. Cantalupo and Hopkins [12] used the cytoarchitectonic map outlined by Bailey et al. [22] to infer the cellular organization of the IFG of chimpanzees and, at the time, no new data were available. Although new data have now been presented by Sherwood et al. [20], the evidence of other cell types present in the IFG does not directly refute the methods used to quantify the gross morphology of this brain region. Moreover, as the same landmarks are used in the left and right hemispheres, this evidence has no effect on the measurement of asymmetry.
Conclusion
Collectively, the morphological differences in the IFG in relation to hand preferences for manual gestures described in this report further support the claim that lateralization in gestures in early Hominids may have played a significant role in the evolution of human language [6]. The results reported here suggest that the common ancestor of humans and chimpanzees may have exhibited a lateralized gestural communication system that was subsequently elaborated upon in Hominid evolution to include motor systems associated with speech production.
Acknowledgments
Sponsorship: This work was supported in part by NIH Grants RR-00165, NS-36605, NS-42867, and 1F32DC007823.
References
- 1.Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- 2.Hickok G, Bellugi U, Klima ES. The neural organization of language: evidence from sign language aphasia. Trends Cogn Sci. 1998;2:129–136. doi: 10.1016/s1364-6613(98)01154-1. [DOI] [PubMed] [Google Scholar]
- 3.Grossi G, Semenza C, Corazza S, Volterra V. Hemispheric specialization for sign language. Neuropsychologia. 1996;34:737–740. doi: 10.1016/0028-3932(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 4.Zahn R, Huber W, Drews E, Erberich S, Krings T, Willmes K, Schwarz M. Hemispheric lateralization at different levels of human auditory word processing: a functional magnetic resonance imaging study. Neurosci Lett. 2000;287:195–198. doi: 10.1016/s0304-3940(00)01160-5. [DOI] [PubMed] [Google Scholar]
- 5.Thiel A, Herholz K, von Stockhausen H-M, van Leyen-Pilgram K, Peitrzyk U, Kessler J, et al. Localization of language-related cortex with 15O-labeled water: PET in patients with gliomas. Neuroimage. 1998;7:284–295. doi: 10.1006/nimg.1998.0334. [DOI] [PubMed] [Google Scholar]
- 6.Corballis MC. From hand to mouth: the origins of language. Princeton University Press; Princeton, NJ: 2002. p. 272. [Google Scholar]
- 7.Leavens DA, Hopkins WD. Intentional communication by chimpanzee (Pan troglodytes): a cross-sectional study of the use of referential gestures. Dev Psychol. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and use of gestural signals by young chimpanzees: a trans-generational study. Primates. 1994;35:137–154. [Google Scholar]
- 9.Bonvillian JD, Richards HC, Dooley TT. Early sign language acquisition and the development of hand preferences in young children. Brain Language. 1997;58:1–22. doi: 10.1006/brln.1997.1754. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins WD, Cantero M. From hand to mouth in the evolution of language: the influence of vocal behavior on lateralized hand use in manual gestures by chimpanzees (Pan troglodytes) Dev Sci. 2003;6:55–61. [Google Scholar]
- 11.Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro S. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychol Sci. 2005;16:487. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantalupo C, Hopkins WD. Asymmetric Broca's area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor cortex but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins WD, Leavens DA. Hand use and gestural communication in chimpanzees (Pan troglodytes) J Comp Psychol. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): evidence of a left hemisphere specialization in motor skill. J Exp Psychol: Gen. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins WD, Russell JL, Hook M, Braccini S, Schapiro SJ. Simple reaching is not so simple: association between hand use and grip preferences in captive chimpanzees. Int J Primatol. 2005;26:259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins WD, Marino L, Rilling JK, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- 19.Lvenbruck H, Baciu M, Segebarth C, Abry C. The left inferior frontal gyrus under focus: an fMRI study of the production of deixis via syntactic extraction and prosodic focus. J Neurolinguistics. 2005;18:237. [Google Scholar]
- 20.Sherwood CS, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca's area homologue in great apes: implication for language evolution. Anat Rec. 2003;217A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- 21.Cantalupo C, Rodes WM, Hegarty JP, Freeman H, Hopkins WD. Macrostructural asymmetry of Broca's area region in the great ape brain: is morphological variability an insurmountable problem? [abstract]; Society for Neuroscience 35th Annual Meeting; Washington, DC. 2005. [Google Scholar]
- 22.Bailey P, von Bonin G, McCulloch WS. The isocortex of the chimpanzee. University of Illinois Press; Urbana-Champaign: 1950. [Google Scholar]



