Abstract
This study evaluated laterality in scratching by chimpanzees (n = 89) during socially arousing circumstances. Hand use and the side of the body scratched was recorded during a baseline and experimental condition. In the experimental condition, chimpanzees were shown a video of other conspecifics sharing, fighting over, and consuming a watermelon. Self-touches were categorized as either rubs or scratches. The chimpanzees showed a significant right hand bias for rubbing and also significantly directed the rubs to the right side of the body. For scratching, the chimpanzees showed no hand preference but a significant bias for scratching on the left side of the body. These results support the view that the right hemisphere regulates the autonomic nervous system during arousal.
Keywords: laterality, chimpanzee, scratching, arousal, emotional contagion
Dating back to the studies on Phineas Gage, there has been significant interest in the potential role of the left and right cerebral hemispheres in the perception and production of emotions (Le-Doux, 1996; Damasio, 2000). Although early studies of asymmetry in behavior and neuroanatomical structures focused on language, subsequent clinical and experimental studies have demonstrated lateralization in emotional processing and production. In humans, the right hemisphere has been strongly linked to emotion perception and production (Borod, Haywood, & Koff, 1997), although some have suggested that the two hemispheres differentially process positive and negative emotions (Davidson, 1992, 1995).
In contrast to humans, consideration of the potential role of the left and right cerebral hemispheres for emotional processing and production in animals has received far less empirical investigation. Indeed, until recently, lateralization of function has been considered hallmark of human evolution (Bradshaw & Rogers, 1993; Corballis, 1992, 2002). However, recent studies in a host of vertebrate species have documented population-level behavioral and neuroanatomical asymmetries (Rogers & Andrew, 2002). With respect to emotion or affect, there have been fewer studies, but the general findings have pointed to homologous right hemisphere asymmetries for emotional responding (reviewed in Rogers & Andrew, 2002). For example, in rats, lesions to the left but not right hemisphere results in increased rates of muricide (Denenberg & Yutzey, 1985). In chicks, testosterone-treated individuals show increased levels of attack and copulatory behavior when tested monocularly using the left eye but not when using the right eye (reviewed in Rogers & Andrew, 2002). Toads will show greater fear responses to a simulated predator when information is presented to the left compared to right visual field (Lippolis, Bisazza, Rogers, & Vallortigara, 2001). Similarly, in fish, right hemisphere advantages have been found for visual inspection of predators (Cantalupo, Bisazza, & Vallortigara, 1995).
In phylogenetically more closely related nonhuman primates, studies of asymmetries in emotional processing have primarily focused on the perception and production of facial expressions. In marmosets, rhesus monkeys and chimpanzees, species-specific facial expressions are expressed more intensely on the left compared to the right side of the face (Fernandez-Carriba, Loches, & Hopkins, 2002; Hook-Costigan & Rogers, 1998; Hauser, 1993). It has also been reported that split-brain monkeys discriminate species-specific facial expression better with the right compared to the left hemisphere (Hamilton & Vermeire, 1988; Vermeire & Hamilton, 1998) and prefer to look at conspecifics with the right compared to left eye (Ifune, Vermeire, & Hamilton, 1984; see also Rogers, Ward, & Stafford, 1994 for visual preference results in bush babies). Physiologically, differences between the EEG power functions of the left and right frontal cortex predicts approach-avoidance in rhesus monkeys (Kalin, Larson, Shelton, & Davidson, 1998) as well as lateralized responses to anxiety medications (Davidson, Kalin, & Shelton, 1992). Similarly, in marmosets, rhesus monkeys and chimpanzees, lateralized changes in tympanic membrane temperature have been associated with responses to different arousal-inducing videos or social circumstances (Boyce, Higley, Jemerin, Champoux, & Suomi, 1996; Parr & Hopkins, 2001; Tomaz, Verburg, Boere, Pianta, & Belo, 2003).
In the current study we sought to examine whether chimpanzees would show asymmetries in scratching behavior that was induced by a mild social stressor. In nonhuman primates there is a substantial amount of literature suggesting that self-directed behaviors are an indicator of anxiety because of uncertainty, social tension, or impending danger [see Aureli & Whiten (2003) for a summary of the relevant literature]. Studies of both captive macaques (Pavani, Maestripieri, Schino, Turillazzi, & Schucchi, 1991; Troisi & Schino, 1987; Maestripieri, 1993) and wild olive baboons (Castles, Whiten, & Aureli, 1999) have found that rates of self-scratching are higher when an individual is in close proximity to a higher-ranking monkey, a time when interindividual aggression is more likely, than when alone or near a subordinate monkey.
Uncertainty has also been shown to increase rates of self-directed behaviors in macaques. For example, intermediate-ranking rhesus macaques scratched themselves at higher rates during feeding times than dominant and subordinate individuals most likely because they experienced higher levels of uncertainty about how to behave during a feeding situation than those with more clear cut social rankings (Diezinger & Anderson, 1986). Higher rates of self-directed behaviors were recorded when unfamiliar long-tail macaque females were introduced than when familiar females were introduced only when the unfamiliar pair was slow to establish a dominance relationship suggesting that the uncertainty of the social situation created higher levels of anxiety and thus higher rates of scratching (Schino, Maestripieri, Scucchi, & Turilazzi, 1990).
Evidence for scratching as an indicator of negative arousal in both social and nonsocial contexts has also been documented for chimpanzees. For example, there are significant differences in rates of scratching in chimpanzees housed either alone or in crowded conditions (Baker & Aureli, 1996, 1997). Similarly, vocalizations from neighboring groups of captive chimpanzees, and the impending risk of aggression that results, has been shown to increase rates of self-directed behaviors in group-living chimpanzees (Baker & Aureli, 1997). Scratching has also been shown to increase during difficult compared to easy cognitive behavioral tasks, particularly following an incorrect response (Itakura, 1993; Leavens, Aureli, Hopkins, & Hyatt, 2001; Leavens, Aureli, & Hopkins, 2004; see Elder & Menzel, 2001, for related findings in an orangutan).
Further evidence supporting the link between scratching and arousal in nonhuman primates comes from pharmacological studies involving anxiety-inducing and anxiety-reducing drugs. For example, administration of an anxiolytic, lorazepam, a drug that reduces anxiety in humans, decreased rates of scratching in long-tailed macaques while an anxiogenic, FG, 7142, had the opposite effect (Schino, Peretta, Taglioni, Monaco, & Troisi, 1996). Taken together, these findings provide adequate support for the use of scratching as an indicator of anxiety or arousal.
In the current study, scratching was examined during baseline observations and during experimental conditions that were designed to elicit increased arousal. In previous studies in which stress was induced by increasing cognitive tasks demands, Leavens et al. (2001) reported that eight chimpanzees scratched more with the right hand under more stressful conditions. In addition, scratching was directed significantly more to the left compared to the right side of the body. Assuming that sensory neurons associated with itch (e.g., Pansky, Allen, & Budd, 1988) are projected contralaterally, these results would suggest a right hemisphere asymmetry in responses to mild stress in chimpanzees. In this study, rather than using cognitive stressors, we used video scenes of chimpanzees engaging in highly arousing social circumstances as a means of inducing scratching. The use of video allowed us to a) test a larger group of chimpanzees and b) to evaluate whether the asymmetries reported by Leavens et al. (2001) generalized to behaviors in response to passive viewing of social arousal rather than directly manipulated task difficulty.
Method
Subjects
Eighty-nine chimpanzees (Pan troglodytes) housed at the Yerkes National Primate Research Center in Atlanta, Georgia, participated in this study. Of the 89 subjects, there were 38 males and 51 females. Rearing history was categorized in one of three ways: mother-reared subjects were reared by their biological mother for at least the first 30 days of life, nursery-reared subjects were hand-reared in the Yerkes nursery prior to their 30th day of life, and wild-caught subjects were captured from their native home in Africa. In this study there were 25 mother-reared (Mean age = 18.56, SD = 9.60), 56 nursery-reared (Mean age = 21.18, SD = 9.79), and 8 wild-caught (Mean age = 41.00, SD = 5.73) subjects.
Video Materials
Video clips were filmed using a Canon® ZR90 digital video camera at Yerkes National Primate Research Center in Atlanta, Georgia. Clips were filmed of two separate chimpanzee social groups after receiving a watermelon. The video included scenes and accompanying vocalizations of chimpanzees in both affiliative and agonistic encounters as they negotiated possession and sharing of the watermelon. Clips were then captured and edited using Roxio Videowave Movie Creator for Windows®. The 30-minute video was then presented to the chimpanzees using a computer system and a 17-inch computer monitor placed on a rolling cart.
Procedure
Hand used and the side of the body scratched was recorded for chimpanzees during two conditions. During the baseline condition, the experimenter located herself outside of the subject’s home cage and recorded all bouts of scratching for a period of 30 minutes. The experimenter sat approximately (2.5 meters) from the mesh of either the inside or outside portion of the subject’s home cage depending on where the subject was likely to spend the most time (for example, if it was raining or cold the observation was done inside.) For the video condition, the experimenter rolled the computer cart in front of the inside portion of the subject’s home cage approximately 3 meters from the mesh. The experimenter then sat down, started the videotape and recorded scratches from a distance of approximately 6 feet. Most subjects stayed in the area where the experimenter was for entire duration of the 30 minutes. However, in order to avoid the possible confounding stress-related effects of being locked inside or separated from their group, no attempt was made to lock animals into a given area during either condition. Thus, animals were free to move into the opposite portion of their cages and to the outdoor portion of their home cage, thus out of view of the experimenter.
The order of presentation of the baseline and experimental conditions was counterbalanced across subjects with approximately half of all subjects receiving the baseline condition followed by the experimental condition and the other half receiving the experimental condition followed by the baseline. A minimum of 12 hours separated the presentation of the two conditions to minimize any carry-over effects of one condition to the other. Testing was carried out between four and six o’clock in the evening, typically a very calm and quiet time of day. If testing was done in the morning, then the experimenters made every effort to do both the baseline and experimental conditions at approximately the same time of day. Scratching was scored three ways including rubs, gentle scratches, and rough scratches [Leavens et al., 2001, 2004]. A rub was defined as a self-touch not involving the ends of the digits. A gentle scratch consisted of a self-touch involving the ends of the digits but no discernable movement of the shoulder joint. Rough scratches were defined as self-touches that involved the ends of the digits and movement of the shoulder joint. The experimenter also recorded the side of the body scratched as left, center, or right. Left-sided and right-sided scratches took place only on the corresponding side of the body or face. A scratch was recorded as occurring in the center of the body if the action of a single scratch extended over both sides of the body or face or if the subject touched only body parts in the mid-line of the body or face (such as rubbing the nose).
Bouts of scratching were recorded during each condition. The onset of a bout was recorded when the subject used one hand to scratch a part of their body or face. A bout of scratching ended in one of three ways: 1) a self-touching event stopped for a period of three or more seconds, 2) the subject switched hands, or 3) the body region being scratched changed.
To ensure that it was the video itself and not the computer cart or testing environment that elicited the self-directed behaviors, a random sample of 16 chimpanzees (8 males, 8 females) were subjected to an additional test for comparison to the baseline and video test conditions. In this manipulation, the computer cart was set up in front of the subjects home cage and a compact disc of nature sounds accompanied by soft music was played. The computer screen displayed the random rhythmic patterns created by Windows Media Player® to accompany the music. As with the initial study, the video was played for 30 minutes, and the frequency of rough and gentle scratches as well as rubs was recorded, and these values were compared to the original baseline and chimpanzee video frequencies.
Data Analysis
The effect of the video on scratching frequency was analyzed in two ways. First, parametric statistics (ANOVA, t-tests) were used to evaluate differences in the number of each of the three types of scratches (rubs, gentle, and rough). Second, all three types of scratches were summed to evaluate the overall increase or decrease in self-touches based on condition using a paired-samples t-test. Handedness indices (HI) were calculated for each category of scratching for the baseline and video condition using the formula (#R − #L)/(#R + #L). Values could range from −1.0 to +1.0 with positive values reflecting greater right hand use and negative values reflecting greater left hand use. Side indices (SI) were also calculated for the three categories of scratching for each condition following the same formula. Positive values indicated more scratching on the right side of the body while negative values reflected greater left-sided scratching. Scratches in the center of the body were not included in the SI calculations.
Results
Experimental Effects on Scratching Frequency
For the initial analysis, a mixed model ANOVA was performed with scratch type (RUB, GS, RS) and condition (baseline, experimental) serving as repeated measures while sex and rearing history served as between group variables. A significant interaction was found between scratch type and condition F(2, 174) = 6.03, p < .001. Subsequent post-hoc tests indicated that the numbers of rubs, gentle and rough scratches were significantly higher in the experimental compared to the baseline condition. In addition, the number of rubs and gentle scratches was significantly higher than the number of rough scratches in the experimental condition (see Table 1). No other significant main effects or interactions were found.
Table 1.
Mean, Standard Error, and t-Value for Each Type of Scratch Across Conditions
| Type of scratch | Baseline | Video | t-value |
|---|---|---|---|
| Rough | 1.93 (.30) | 5.06 (.74) | 4.263* |
| Gentle | 5.76 (.84) | 13.88 (1.48) | 5.399* |
| Rub | 4.67 (.42) | 11.13 (1.20) | 5.355* |
| Total scratches | 12.37 (1.02) | 30.07 (2.47) | 7.191* |
p < .001.
To test whether the video per se induced scratching or the presence of the computer cart and experimental set up, t-tests were performed between the baseline and video conditions to the music baseline condition. A paired-samples t-test revealed no significant difference between the frequency of scratches elicited by the new music baseline condition (M = 11.06, SE = 2.27) and the previous baseline condition (M = 14.44, SE = 3.09) for those 16 subjects tested in this condition. In contrast, subjects scratched significantly more during the initial experimental condition (M = 29.77, SE = 5.01) than during the music baseline condition (M = 11.06, SE = 2.27) t(15) = 3.17, p < .01.
Laterality Effects
Because of the relatively low occurrence of rough scratches (see Table 1), gentle and rough scratches were combined and referred to as scratches for the subsequent laterality analyses. Because hand use for scratching and the body side to which the scratching was directed were not independent events, for these analyses, we combined the frequencies in responding into four categories including left-hand, left-side (LH-LS), left-hand, right side (LH-RS), right-hand, left-side (RH-LS) and right-handed, right-side (RH-RS). We then compared the frequency of occurrence of each of the four behavioral categories for rubs and scratches using a mixed model ANOVA. For scratching F(3, 264) = 24.18, p < .001 and rubs F(3, 264) = 9.24, p < .001, significant differences in frequency were found for the response categories. The mean number of responses for each hand and body side combination for rubs and scratches are shown in Figure 1. Post-hoc analysis of the scratching results indicated that the chimpanzees produced significantly more responses in the RH-LS conditions compared to all other conditions. In addition, subjects produced more responses in the LH-RS condition compared to the LH-LS and RH-RS conditions. No significant difference was found between the LH-LS and RH-RS conditions. Thus, most scratches were directed contralaterally to the scratching hand. For rubs, post-hoc analysis indicated that subjects made significantly more RH-RS responses compared to the LH-RS and LH-LS conditions but not the RH-LS condition. The chimpanzees also produced significantly more RH-LS responses than in the LH-RS and LH-LS conditions. No significant differences were found between the LH-LS and LH-RS responses. We also compared the frequency of occurrence of each of the four categories of responses for rubs and scratches using a series of paired t-tests. The chimpanzees produced significantly more scratches than rubs in the LH-LS t(88) = 4.088, p < .01, LH-RS t(88) = 6.32, p < .01 and RH-LS t(88) = 6.52, p < .01 conditions but not in the RH-RS condition.
Figure 1.
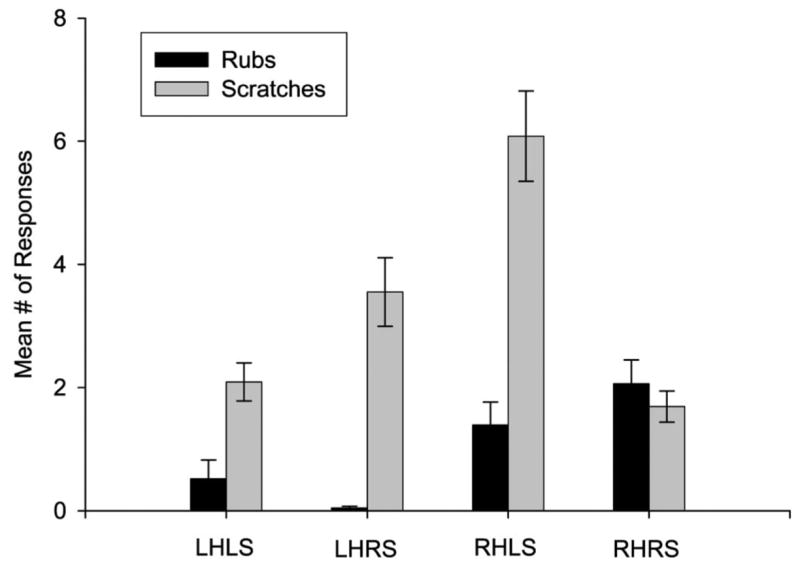
Mean number of rubs and scratches with standard errors for each of the four possible lateralized responses: left hand/left side, left hand/right side, right hand/left side, and right hand/right side.
As an alternative means of evaluating laterality in scratching, we performed one sample t-tests on the HI and SI scores that were calculated for rubs and scratches. This analysis was done to assess whether the asymmetries deviated significantly from zero, which would be predicted if the lateralized responses were normally or bimodally distributed. For rubs, significant population-level rightward asymmetries were found for the HI t(88) = 3.90, p < .01 and SI t(88) = 3.78, p < .01 (see Figure 2). In contrast, for scratching, significant leftward asymmetries were found for the SI scores t(88) = −3.84, p < .01 but not the HI scores (see Figure 2).
Figure 2.
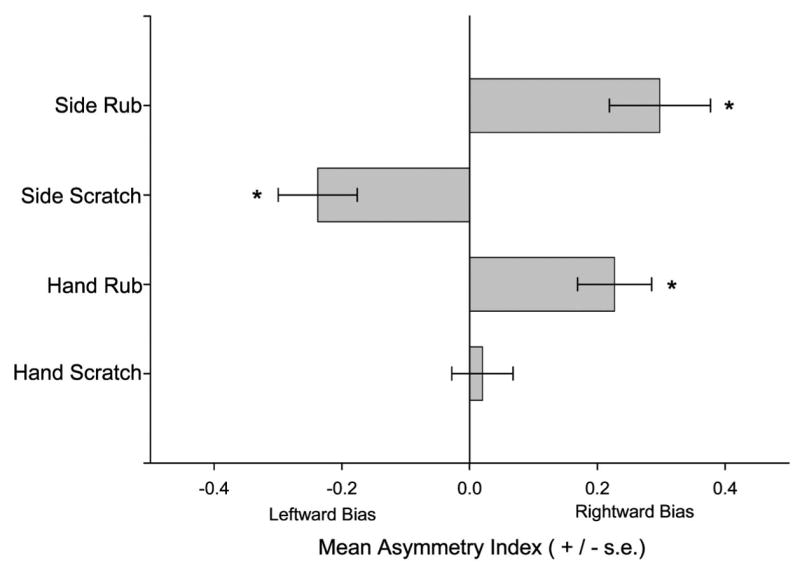
Mean asymmetry indices and standard errors for the hand used to rub, the side of the body rubbed, the hand used to scratch, and the side of the body scratched.
Discussion
Two significant findings were revealed in this study. First, consistent with previous research in nonhuman primates, increased rates of self-directed behaviors were found in response to a stressful stimulus. Second, laterality in self-directed behaviors was evident in chimpanzees but was somewhat influenced by the type of self-directed response. No sex differences in self-directed behaviors were evident in either the baseline or experimental condition.
The comparison of scratching rates in the social video condition compared to the baseline and music baseline conditions indicates that use of the social scenes depicted in the videotapes are salient enough to induce arousal in the chimpanzees, as evidenced by the patterns of self-directed behaviors, and these results were not because of factors associated with the experimental procedure or the use of novel visual and auditory stimuli. In previous research with chimpanzees (e.g., Baker & Aureli, 1997), inducing social stress has always been done in natural contexts rather than through use of video scenes. Thus, our results are the first to demonstrate that increased self-directed behavior can be induced in chimpanzees by showing social interactions of chimpanzees on a computer monitor, which we interpret to demonstrate contagion of arousal (see Nakayam, 2004, for a similar example in Japanese macaques, Macaca fuscata).
With respect to the laterality effects in self-directed behaviors, a different pattern of use was seen for rubs compared to scratches. For rubs, the chimpanzees preferred to use their right hand and showed a rightward (ipsilateral) bias for the side of the body rubbed. In contrast, for scratching, there was no strong manifestation of hand use but a more strongly lateralized response to the location where the scratch was directed, notably the left side of the subject’s body. Indeed, scratching the left side of the body with the right hand was the most frequently observed response during the experimental condition. These results are largely consistent with the previous findings reported by Leavens et al. (2001) in a much smaller cohort of chimpanzees and somewhat consistent with previous findings in wild chimpanzees (Marchant & McGrew, 1996; McGrew & Marchant, 2001). We believe the most parsimonious explanation for these findings is that mediation of arousal is controlled by the right hemisphere of chimpanzees. Studies in humans and rats have clearly implicated the medial prefrontal cortex (and other brain regions) of the right hemisphere as mediating autonomic nervous systems responses to stress (e.g., Denenberg & Yutzey, 1985; Meador, Ray, Day, Ghelani, & Loring, 1998), and our results are consistent with this body of work. Thus, the viewing of highly animated social interactions in conspecifics presumably activates the autonomic nervous system such that the left hemispace of the body becomes preferentially targeted for self-directed behaviors. Leavens et al. (2001, 2004) have speculated that a right-hemisphere specialization for negative emotion may modulate cutaneous sensation differentially across the left and right sides of the body. Specifically, they suggested that ipsilateral descending inhibition of primary cutaneous afferents responsible for pain and itch may be manifested more strongly in the right dorsal horn, compared to the left, under conditions of negative arousal, because of the functional asymmetry expressed in emotional processing in limbic and, possibly, higher cortico-limbic areas. Alternatively, negative arousal may result in more direct and lateralized autonomic responses, such as piloerection or, possibly, histamine release and the proximate cause of the RH-LS dominant pattern of scratching reported here may be a series of cutaneous sensations directly caused by autonomic activity. Because autonomic control of piloerection is largely ipsilateral and because we believe these lateral asymmetries ultimately stem from a right-hemisphere specialization for negative emotion, we would expect to see a more dominant pattern of LH-RS scratching, and for this reason we favor the view that there is an asymmetry in perception of relatively symmetrical cutaneous stimulation, but the present state of knowledge permits several neurophysiological models, and more research in this area is therefore warranted. Either way, a left side bias in physiological activity relating to cutaneous sensation is implicated by the pattern depicted in Figure 2, in which a significant shift to left-hemispace-directed scratches in the absence of a concomitant change in handedness is depicted.
The distinction between the types of lateralized responses observed in the chimpanzees for rubs and scratches likely reflects where the responses are directed on the body. Although no distinction was made in the location of self-directed responses in the vertical dimension in this study, Leavens et al. (2004) have previously shown that rubs are largely directed to the face and head whereas scratches are largely directed to the body, demonstrating an association between rubs and parts of the face subserved by the trigeminal nerve and a concomitant association between scratching and the regions of the skin subserved by the spinothalamic trunk. This was the general impression we had of the chimpanzees in this study, and it would make sense that hard, potentially painful scratching responses would not be made on the face, a much more sensitive region.
The results of this study also differ from a somewhat curious set of studies on laterality in self-touching by human and nonhuman primates (Dimond & Harries, 1984; Hopkins & de Waal, 1995; Rogers & Kaplan, 1995; Shafer, 1993, 1997). Laterality of hand use for self- or face-touching has been reported in a number of research articles describing spontaneous hand use in wild and captive monkeys and apes. Overall, there have been no consistent findings across studies or species which calls into question the validity of the measure as an indicator of hemispheric specialization. The present study, considered in relation to previous experimental studies (Leavens et al. 2001, 2004), suggests that it is the change in laterality across conditions of differential arousal or mild negative emotion that elicits consistent patterns of asymmetry in self-directed behavior rather than hand use for spontaneous, self-directed manual actions.
Whether asymmetries observed in captive populations of primates generalize to wild subjects remains a topic of considerable debate (McGrew & Marchant, 1997; Hopkins & Cantalupo, 2004; Palmer, 2002). Hopkins and Cantalupo (2004) have argued that comparing laterality findings in wild and captive apes is difficult because very often different measures of hand use are used in these various settings. Scratching, particularly in response to natural social stressors (see van Lawick-Goodall, 1972), is frequently seen in wild chimpanzees and measuring this behavior would provide an ideal means of assessing asymmetry in stress responses in both captive and wild apes. Marchant and McGrew (1996) and McGrew and Marchant (2001) have recorded hand use for scratching and nose wiping (perhaps like rubs) in two populations of wild chimpanzees. The results were not consistent between the two populations but combining the data indicates that there were more right-than left-handed subjects for scratching and nose wiping. No attempt was made to characterize the social circumstance or the side to which the scratching was directed, but this would be an excellent opportunity for further investigation in wild apes and other nonhuman primates.
In sum, these results affirm previous studies in chimpanzees showing asymmetries in self-directed behaviors in relation to increasing arousal but demonstrate them in a much larger cohort of apes as well as in response to a social stressor, scenes of chimpanzees, rather than a cognitive stressor, a difficult computer task. The results generally support the view that the right hemisphere regulates autonomic nervous system response to arousal, particularly negative arousal as has been reported in humans and rats. The extent to which lateralized scratching predicts physiological indicators of stress such as heart rate, skin conductance or cortisol responses remains unknown but could be valuable line of inquiry (cf. Elder & Menzel, 2001). Collectively the findings add to a growing body of literature on hemispheric specialization in animals (Rogers & Andrew, 2002), and further research should provide important information on social and evolutionary factors that influence behavioral and brain asymmetries of primates, including humans.
Acknowledgments
This work was supported in part by NIH grants RR-00165, NS-42867, NS-36605 and HD-38051.
Footnotes
All APA guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
References
- Aureli F, Whiten A. Emotions and behavioral flexibility. In: Maestripieri D, editor. Primate psychology: The mind and behaviour of human and nonhuman primates. Cambridge, MA: Harvard University Press; 2003. pp. 289–323. [Google Scholar]
- Baker KC, Aureli F. The neighbor effect: Other groups influence intra-group agonistic behavior in captive chimpanzees. American Journal of Primatology. 1996;40:283–291. doi: 10.1002/(SICI)1098-2345(1996)40:3<283::AID-AJP5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Baker KC, Aureli F. Behavioural indicators of anxiety: An empirical test in chimpanzees. Behaviour. 1997;134:1031–1050. [Google Scholar]
- Borod JC, Haywood CS, Koff E. Neuropsychological aspects of facial asymmetry during emotional expression: A review of the normal adult literature. Neuropsychology Review. 1997;7:41–60. doi: 10.1007/BF02876972. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Higley JD, Jemerin JJ, Champoux M, Suomi SJ. Tympanic temperature asymmetry and stress behavior in rhesus macaques and children. Archives of Pediatric and Adolescent Medicine. 1996;150:518–523. doi: 10.1001/archpedi.1996.02170300072014. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Rogers L, editors. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Cantalupo C, Bisazza A, Vallortigara G. Lateralization of predator-evasion response in a teleost fish (Girardinus falcatus) Neuropsychologia. 1995;33:1637–1646. doi: 10.1016/0028-3932(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Castles DL, Whiten A, Aureli F. Social anxiety, relationships, and self-directed behavior among wild female olive baboons. Animal Behaviour. 1999;58:1207–1215. doi: 10.1006/anbe.1999.1250. [DOI] [PubMed] [Google Scholar]
- Corballis MC, editor. The lopsided brain: Evolution of the generative mind. New York: Oxford University Press; 1992. [Google Scholar]
- Corballis MC. From hand to mouth: The origins of language. Princeton: Princeton University Press; 2002. [Google Scholar]
- Damasio AR. Cognition, emotion and autonomic responses: The integrative role of the prefrontal cortex and limbic structures. Amsterdam: Elsevier Science Bv; 2000. The fabric of the mind: A neurobiological perspective; pp. 457–467. [Google Scholar]
- Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychological Science. 1992;3:39–43. [Google Scholar]
- Davidson RJ. Cerebral asymmetry, emotion and affective style. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge, MA: MIT Press; 1995. pp. 361–387. [Google Scholar]
- Davidson RJ, Kalin NH, Shelton SE. Lateralized effects of diazepam on frontal brain electrical asymmetries in rhesus monkeys. Behavioral Neuroscience. 1992;32:438–451. doi: 10.1016/0006-3223(92)90131-i. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Handedness hangups and species snobbery. Behavioral and Brain Sciences. 1988;11:721–722. [Google Scholar]
- Denenberg VH, Yutzey DA. Hemispheric laterality, behavioral asymmetry, and the effects of early experience in rats. In: Glick SD, editor. Cerebral lateralization in nonhuman species. New York: Academic Press; 1985. pp. 109–133. [Google Scholar]
- Diezinger F, Anderson JR. Starting from scratch: A first look at “displacement activity” in group-living rhesus monkeys. American Journal of Primatology. 1986;11:117–124. doi: 10.1002/ajp.1350110204. [DOI] [PubMed] [Google Scholar]
- Dimond S, Harries R. Face touching monkeys, apes and man. Evolutionary origins and cerebral asymmetry. Neuropsychologia. 1984;22:227–233. doi: 10.1016/0028-3932(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Elder CM, Menzel CR. Dissociation of cortisol and behavioral indicators of stress in an orangutan (Pongo pygmaeus) during a computerized task. Primates. 2001;42:345–358. [Google Scholar]
- Fernandez-Carriba S, Loeches A, Morcillo A, Hopkins WD. Asymmetry of facial expression of emotions by chimpanzees. Neuropsychologia. 2002;40:1523–1533. doi: 10.1016/s0028-3932(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Hamilton CR, Vermeire BA. Complimentary hemispheric specialization in monkeys. Science. 1988;242:1694–1696. doi: 10.1126/science.3201258. [DOI] [PubMed] [Google Scholar]
- Hauser MC. Right hemisphere dominance in the production of facial expression in monkeys. Science. 1993;261:475–477. doi: 10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Lateralized use of the mouth in production of vocalizations by marmosets. Neuropsychologia. 1998;36:1265–1273. doi: 10.1016/s0028-3932(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): A critical analysis and some alternative explanations. Laterality. 2004;10:65–80. doi: 10.1080/13576500342000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, de Waal FD. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. International Journal of Primatology. 1995;16:261–276. [Google Scholar]
- Ifune CK, Vermeire BA, Hamilton CR. Hemispheric differences in split-brain monkeys and responding to videotape recordings. Behavioral and Neural Biology. 1984;41:231–235. doi: 10.1016/s0163-1047(84)90639-3. [DOI] [PubMed] [Google Scholar]
- Itakura S. Emotional behavior during the learning of a contingency task in a chimpanzee. Perceptual and Motor Skills. 1993;76:563–566. doi: 10.2466/pms.1993.76.2.563. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behavioral Neuroscience. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD. Behavioral evidence for the cutaneous expression of emotion in a chimpanzee (Pan troglodytes) Behaviour. 2004;141:979–997. [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD, Hyatt CH. The effects of cognitive challenge on self-directed behaviors by chimpanzees (Pan troglodytes) American Journal of Primatology. 2001;55:1–14. doi: 10.1002/ajp.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, editor. The emotional brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Lippolis G, Bisazza A, Rogers LJ, Vallortigara G. Lateralisation of predator avoidance responses in three species of toads. Laterality. 2002;7:163–183. doi: 10.1080/13576500143000221. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta): I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993;95:19–31. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- Meador KJ, Ray PG, Day L, Ghelani H, Loring DW. Physiology of somatosensory perception: Cerebral lateralization and extinction. Neurology. 1998;51:721–727. doi: 10.1212/wnl.51.3.721. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Observing conspecifics scratching induces a contagion of scratching in Japanese monkeys (Macaca fuscata) Journal of Comparative Psychology. 2004;116:20–24. doi: 10.1037/0735-7036.118.1.20. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: Evaluating the evidence with funnel plots. American Journal of Physical Anthropology. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Pansky B, Allen DJ, Budd GC. Review of Neuroscience. 2. New York: Macmillan; 1988. [Google Scholar]
- Parr LA, Hopkins WD. Brain temperature asymmetries and emotional perception in chimpanzees, Pan troglodytes. Physiology and Behavior. 2001;71:363–371. doi: 10.1016/s0031-9384(00)00349-8. [DOI] [PubMed] [Google Scholar]
- Pavani S, Maestripieri D, Schino G, Turillazzi PG, Schucchi S. Factors influencing scratching behavior in long-tail macaques. Folia Primatologica. 1991;57:34–38. [Google Scholar]
- Rogers LJ, Andrew JR. Comparative vertebrate lateralization. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans, Pongo pygmaeus pygmaeus. Animal Behaviour. 1995;51:13–25. [Google Scholar]
- Rogers LJ, Ward JP, Stafford D. Eye dominance in the small-eared bushbaby, Otolemur garnetti. Neuropsychologia. 1994;32:257–264. doi: 10.1016/0028-3932(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Schino G, Maestripieri D, Scucchi S, Turilazzi PG. Social tension in familiar and unifamiliar pairs of long-tail macaques. Behaviour. 1990;113:264–272. [Google Scholar]
- Schino G, Peretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Shafer DD. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. pp. 267–283. [Google Scholar]
- Shafer DD. Hand preference behaviors shared by two groups of captive bonobos. Primates. 1997;38:303–313. [Google Scholar]
- Tomaz C, Verburg MS, Boere V, Pianta TF, Belo M. Evidence of hemispheric specialization in marmosets (Callithrix peni-cillata) using tympanic membrane thermometry. Brazilian Journal of Medical and Biological Research. 2003;36:913–918. doi: 10.1590/s0100-879x2003000700012. [DOI] [PubMed] [Google Scholar]
- Troisi A, Schino G. Environmental and social influences on autogrooming behavior in a captive group of Java monkeys. Behaviour. 1987;100:292–302. [Google Scholar]
- van Lawick-Goodall J. A preliminary report on expressive movements and communication in the Gombe Stream chimpanzees. In: Dolhinow P, editor. Primate patterns. New York: Holt, Rinehart, & Winston; 1972. pp. 25–84. [Google Scholar]
- Vermeire BA, Hamilton CR. Inversion effect for faces in split-brain monkeys. Neuropsychologia. 1998;36:1003–1014. doi: 10.1016/s0028-3932(98)00054-2. [DOI] [PubMed] [Google Scholar]


