Abstract
Gyrification of the cerebral cortex reflects complexity in cortical folding during development of the brain. In this paper, we evaluated whether chimpanzees show asymmetries in gyrification and if variation in gyrification asymmetries were associated with handedness. Magnetic resonance images were obtained in a sample of 76 chimpanzees, and gyrification measures were obtained from 10 equally spaced slices of the cortex. Asymmetry quotients (AQs) in gyrification were compared for 4 measures of handedness including reaching, coordinated bimanual actions, manual gestures, and throwing. Overall, the chimpanzees showed significant differences between the right and left hemispheres that were region specific. Significant differences in AQ's were found in right- and nonright-handed chimpanzees for throwing and, to a lesser degree, for manual gestures. Increasing age was associated with increasing gyrification in the prefrontal regions, particularly in female chimpanzees. The results indicate that variation in gyrification between hemispheres is associated with functional measures of laterality in chimpanzees.
Keywords: asymmetry, chimpanzees, gyrification, handedness
Introduction
Comparative organization of the central nervous system of different primate species has been a historical and contemporary focus of research in anthropology, psychology, biology, and the neurosciences. Allometric variation in the cellular and morphological characteristics has been described in different primates as a framework for understanding evolutionary changes in the brain relative to other morphological features (Jerison 1973; Passingham 1982; Preuss 2000; Rilling 2006). For example, it is well known that brain size increases in primates relative to body size, with humans having a brain that is 3 times larger than would be predicted for an organism of our body size (Stephan et al. 1981). In addition to overall brain size, studies have also examined regional changes in brain organization in primates. For example, researchers have examined the relative size of the different lobes of the brain as well as ratios of white to gray matter in different primate species (Deacon 1997; Semendeferi and Damasio 2000; Rilling and Seligman 2002; Schoenemann et al. 2005 but see Sherwood et al. 2005). Others have evaluated the relative change in the size of specific brain regions, such as the corpus callosum or cerebellum, in different primates (Rilling and Insel 1998, 1999a). Lastly, the degree of gyrification of the brains of primates varies with primates more closely related to humans having greater gyrification compared with more distantly related primate species (Rilling and Insel 1999b).
Gyrification reflects the magnitude of cortical folding in a brain. As brains became larger via evolutionary changes, they were increasingly constrained by the size of the cranium. Therefore, the outer cortical tissue folded inward to create sulci and gyri in various regions of the brains (Zilles et al. 1988). In humans and other primates, there is a general anterior-to-posterior gradient in gyrification with lower values toward the rostral portion of the brain (Zilles et al. 1989). Developmental studies suggest that cortical folding develops by 6 months of age and that gyrification measurements are a consequence of programmed maturation of the cortex (Rakic 1988; Armstrong et al. 1993). Clinical studies in schizophrenic and autistic patients have revealed atypical patterns of gyrification, particularly between the 2 hemispheres, and these results may reflect early problems in neurogenesis for the developing fetus, making some individuals more prone to illness (White et al. 2003; Casanova et al. 2004; Harris et al. 2004).
The specific purpose of this paper was to evaluate whether 1) there are asymmetries in the gyrification of chimpanzee brains and 2) whether the handedness of the chimpanzees has an influence on gyrification patterns. In humans, as far as we know, there are no studies that have directly assessed the influence of handedness on gyrification; however, general descriptive data on individual differences in asymmetries of gyrification have been provided. For example, Zilles et al. (1988) reported general gyrification data on humans and reported no asymmetries, but our own analysis of their raw data did reveal a small but significant difference between the gyrification indices of the left and right hemispheres. No hand preference data of the participants were provided in the Zilles et al. Other studies that have selected subjects on the basis of clinical diagnosis or other factors have self-selected largely right-handed subjects only, thus it is difficult to infer any potential effects of handedness on asymmetries in gyrification. When considering studies of gyrification in nonhuman primates (Rilling and Insel 1999b) or other mammals (Mayhew et al. 1996; Wosinski et al. 1996), there have been no reports of population-level asymmetries. However, these studies were limited to relatively small samples of individuals within a species. Thus, the question of whether asymmetries in gyrification are present in other species remains largely unanswered.
In contrast to studies on gyrification, recent studies in chimpanzees have reported an association between hand use and asymmetries in specific regions of interest. For example, in chimpanzees, Hopkins and Cantalupo (2004) found that right- and left-handed chimpanzees differed in neuroanatomical asymmetries for a region within the primary motor cortex but not for the planum temporale or fronto-orbital sulcus. Moreover, Hopkins and Cantalupo (2004) found that neuroanatomical asymmetries were specific to some measures of hand use but not others. In contrast, Taglialatela et al. (2006) found that variation in asymmetries in the frontal operculum were associated with hand preferences for manual gestures. Thus, the link between behavioral and brain asymmetries in chimpanzees is task and region specific. Based on the previous results on differences in brain asymmetry in right- and left-handed chimpanzees, it was hypothesized that differences in gyrification between the left and right cerebral hemispheres might similarly be present in chimpanzees depending on their handedness. Moreover, it was further hypothesized that any potential differences in gyrification asymmetries might be sensitive to the type of task and possibly region specific. To test these hypotheses, gyrification indices of the left and right hemispheres measured from magnetic resonance images were compared in right- and non-right-handed chimpanzees on 4 different measures of hand use. In addition to the question of handedness and gyrification asymmetry, we further examined the associations between age and brain volume with gyrification indices. In comparative studies, increasing brain size is associated with increasing gyrification (Rilling and Insel 1999b); however, within species, significant associations were found between brain weight and gyrification in dogs (Wosinski et al. 1996) but not in rhesus monkeys (Armstrong et al. 1991). In the current study, we examined whether brain volume is associated with gyrification in chimpanzees.
Methods
Subjects
Subjects were a sample of captive chimpanzees housed at the Yerkes National Primate Research Center (YNPRC). In total, there were 76 subjects including 42 females and 34 males. With the sample, there were 18 mother-reared, 45 human-reared, and 13 wild-caught apes. Mother-reared subjects were apes that had been raised by their conspecific mother. Nursery-reared apes were chimpanzees that were raised by humans. Wild-caught subjects were apes that had been captured in the wild and subsequently raised in captivity. The sample ranged in age from 6 to 45 years (mean = 21.18, standard deviation = 10.99). Magnetic resonance imaging (MRI) scans were collected from both alive apes and cadaver specimens. Within the sample, in vivo MRI scans were collected in 54 while 22 cadaver brains were scanned. The cadaver brains had been fixed in formalin for varying time periods ranging from 5 years to 7 days.
Image Collection and Procedure
Prior to scanning, the subjects were immobilized with ketamine injection (2−6 mg/kg) and subsequently anesthetized with propofol (10 mg/kg/h) following standard veterinary procedures used at the YNPRC. The subjects remained sedated for the duration of the scans as well as the time needed for transport between YNPRC and scanner location (total time approximately 1.5−2 h). After completing the MRI scan, the nonhuman primate subjects were returned to YNPRC and were temporarily singly housed for 6−12 h to allow the effects of the anesthesia to wear off before being returned to their home cage and cage mates.
At the MRI facility, the living animals were placed in the scanner chamber and their heads were fitted inside the head coil. The cadaver brains were placed inside the human knee coil with the dorsal side up. Scan duration ranged between 40 and 80 min as a function of brain size. This project involved using 2 MRI machines (Phillips, Model NT), each with 1.5-T superconducting magnets. For all living subjects, T1-weighted images were collected in the axial plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, slice thickness 1.2 mm, slice overlap = 0.6 mm, number of signals averaged = 8, and a 256 × 256 matrix). Similar scan parameters were used for the cadaver brains, but the images were T2 weighted to increase contrast in gray-white matter. Scan parameters for the T1 and T2 images were based on preliminary studies and provided excellent resolution of the brain areas of interest. The raw images were reformatted into the different planes using multiplanar formatting software (ANALYZE, Mayo Foundation, Rochester, MN).
Gyrification Measurement
The MRI scans were aligned in the coronal planes and cut into 1 mm slices. Consistent with previous studies (Rilling and Insel 1999b), prior to measurement, the maximum length of the left and right hemispheres from the frontal to occipital pole was identified. Once the length was determined, the value was divided by 11, which resulted in 10 equally spaced slices along the anterior-to-posterior plane (see Fig. 1a). For each of these slices and hemispheres, 2 measurements were obtained. First, the entire contour of the slice, including the sulci, was traced with a freehand tool. Second, the perimeter of the entire slice was traced with the freehand tool. Dividing the first contoured slice by the perimeter produces an index of gyrification for each slice (see Fig. 1b). The same procedure was repeated for each of the 10 slices.
Figure 1.
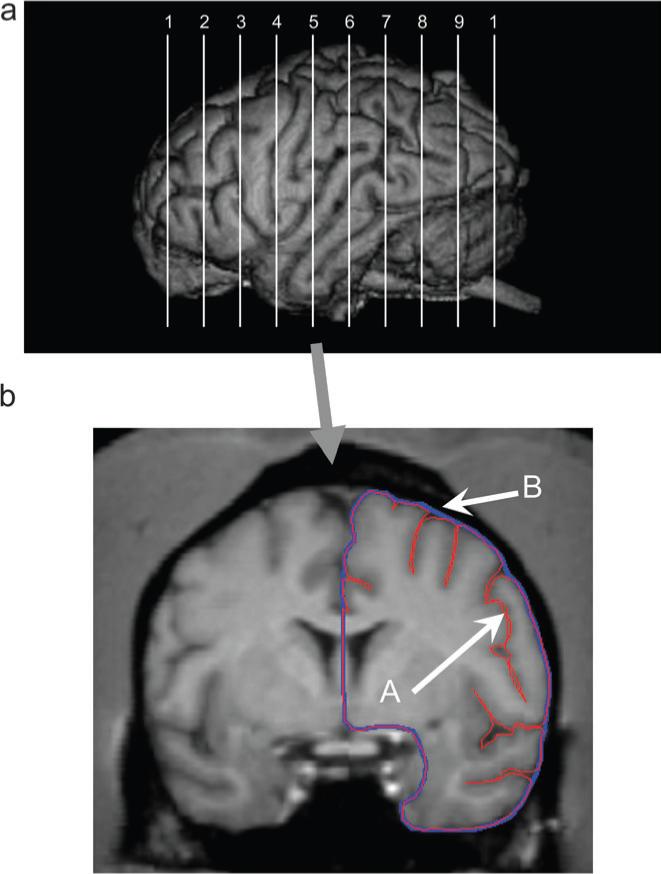
(a) Upper panel shows the approximate locations in the anterior-to-posterior plane from which the 10 slices and gyrification measures were obtained from each subject. (b) The lower panel shows a single 1-mm slice oriented in the coronal plane on which the contour (A, in red) and outer edge (B, in blue) of one hemisphere are traced using ANALYZE.
Individuals tracing the brains were blind to the sex and handedness of the chimpanzees. To assess interrater reliability, 2 individuals measured all 10 brain slices for 3 individual chimpanzees. The area measures of the left and right hemispheres for the outer contour and sulci were correlated within each individual chimpanzee between the 2 raters. For the outer contour, the interrater reliabilities for the left (range = 0.94−0.99) and right hemispheres (range = 0.98−0.92) were all positive and significant. Similarly, for the sulci, the interrater reliabilities for the left (range = 0.85−0.95) and right hemispheres (range = 0.84−0.97) were all positive and significant. Thus, good interrater reliability was obtained in the measurements of the brain areas used to derive the gyrification indices.
Brain Volume
Brain volume was estimated from each scan using automated functions available in the object-extractor and volume-render modules of ANALYZE (Mayo Clinic). Volume measures incorporated the volume of the cerebral hemispheres and brain stem, excluding the cerebellum.
Behavioral Measures of Handedness
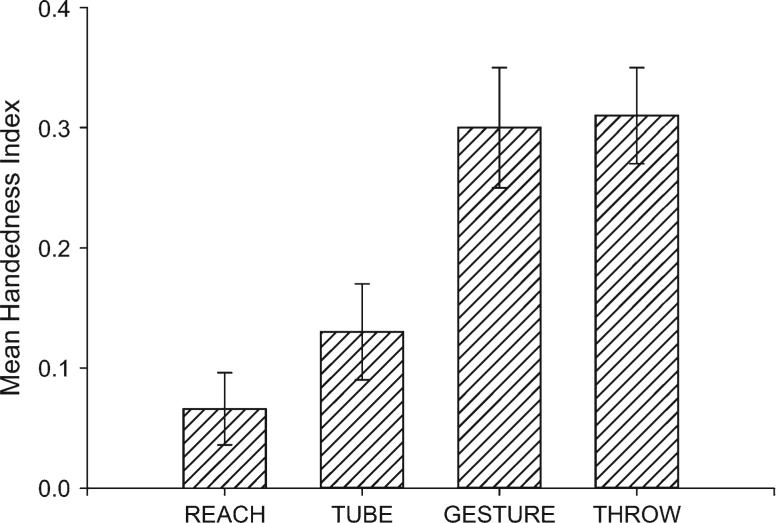
Gyrification measurements were compared in subjects on 4 different measures of hand use including throwing, manual gestures, coordinated bimanual actions (referred to as TUBE), and simple reaching. These 4 measures were selected because previous studies have shown that they induce different degrees of asymmetry in chimpanzees. Shown in Figure 2 are the mean handedness index values for each of the 4 measures. Captive chimpanzees show population-level right handedness for all 4 measures, but the apes are more right handed for throwing and manual gestures compared with the TUBE and reaching tasks. Thus, we were interested in directly testing whether measures that induce a lesser or greater degree of handedness have an influence on variation in asymmetries in gyrification. A brief description of each task is provided below but details of each measure are provided in previous publications (Hopkins et al. 2002, 2004, Hopkins, Russell, Freeman, Buehler, et al., 2005; Hopkins, Russell, Cantalupo, Freeman, et al., 2005; Hopkins 2006).
Figure 2.
Mean handedness index and standard error scores for the 4 behavioral measures of handedness. Positive values reflect right handedness, and negative values reflect left handedness.
Simple Reaching (REACH)
Simple reaching was measured by throwing a raisin into the subject's home cage (for description, see Hopkins et al. 2002). The subjects had to locomote to a spot near the food, reach for, and pick up the food. Hand use was recorded as right or left. Fifty responses were recorded from each subject. To assure that hand choice on each trial was not influenced by the hand used on the previous trial (McGrew and Marchant 1997), subjects had to reposition themselves between trials. All subjects were tested in the outdoor portion of their home cage.
Coordinated Bimanual Actions (TUBE)
The second handedness measure was a task requiring bimanual coordinated actions, referred to as the TUBE task (Hopkins 1995). For the TUBE task, peanut butter is smeared on the inside edges of polyvinyl chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths but rather must use their fingers to remove the substrate. The PVC tubes were handed to the subjects in their home cages, and a focal sampling technique was used to collect individual data from each subject. The hand of the finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter, and brought it to their mouth, the hand used was recorded as left or right.
Manual Gesture (GESTURE)
At the onset of each trial, the experimenter would approach the subject's home cage and offer a food item. Food items included pieces of carrot, bananas, or candy. The experimenter stood approximately 1−1.5 m from the cage and was positioned as much as possible in the center plane of the subject. If not immediately positioned in front of the experimenter, the chimpanzee would approach the front of the cage once the human with the food had arrived. The experimenter would say the chimpanzees name and offer the food to them until a manual gesture was produced by the subject. If by chance the chimpanzee was already gesturing toward the experimenter when they arrived, no response was recorded. Hand use was recorded as left or right. Although rare in occurrence, 2-handed gestures were not scored. In addition, the experimenter noted whether or not the chimpanzee vocalized while gesturing. Subjects were tested over a 15-day period, and the goal was to obtain a minimum of 30 responses from each subject. The number of trials administered per day varied in accordance with subject motivation and availability for testing. Subjects were tested in both the indoor and outdoor sections of their home cages. None of the subjects were separated from their groups or cage mates for the purposes of testing.
Throwing (THROW)
For the past 15 years, observations of hand use for throwing have been obtained in the chimpanzees housed at the YNPRC using all occurrence ad libitum sampling (see Hopkins et al. 2005). Throwing is an infrequent behavior and is most often observed when subjects throw materials from their home cages at strangers in proximity to their cages. When throwing was seen, observers noted the hand used and the posture of the subject. Only subjects that were observed on at least 6 occasions were included in these analyses. A minimum of 6 observations was necessary to obtain a binomial z-score that could be used to classify the handedness of the chimpanzees.
Data Analysis
For each slice and hemisphere, a gyrification index (GI) ratio score was calculated following the procedure used by others (Rilling and Insel 1999b) that involved dividing the internal line by the contour line. The overall GI score for each hemisphere (SUM-GI) was derived by averaging the GI scores for each of the 10 slices. The overall asymmetry in GI scores (SUM-AQGI) was derived by subtracting the left GI scores from the right GI scores and dividing by the total SUM-AQGI = (R – L)/(R + L). SUM-AQGI scores varied on a continuum with positive values reflecting right hemisphere biases and negative values reflecting left hemisphere biases. The absolute value of the SUM-AQGI scores reflected the magnitude of asymmetry. Individual GI and AQGI scores were also derived for each of the 10 slices following the formula described above.
For the 4-handedness tasks, binomial z-scores were calculated for each subject based on the frequency of left and right hand use for all assessments of hand use. Subjects with z-scores greater than 1.95 were classified as right handed, whereas chimpanzees with values ≤1.95 were classified as left handed. All other subjects were classified as ambiguously handed. We subsequently collapsed the left- and ambiguously handed chimpanzees because there were few and in some cases no ambiguously or left-handed individuals. All analyses adopted an alpha of P < 0.05 as the level of significance. Post hoc tests, when necessary, were conducted using Tukey's honestly significant difference with P < 0.05.
Results
Descriptive Statistics
We initially compared the SUM-GI and regional GI values between the cadaver and in vivo specimens to assess whether the brain sample influenced the gyrification results. No significant differences were found. The mean SUM-GI scores for the cadaver and in vivo specimens were 1.86 and 1.85, respectively. Because there was no difference in the gyrification values between cadaver and in vivo specimens, we combined the data for all subsequent analyses. We also compared the SUM-GI values between sexes and rearing conditions using an analysis of variance. No significant main effects or interactions were found. The mean SUM-GI values as a function of specimen type, sex, and rearing history are shown in Table 1.
Table 1.
Mean SUM-GI values as a function of specimen type, sex, and rearing history
| Mean | SE | N | |
|---|---|---|---|
| Specimen type | |||
| Cadaver | 18.49 | 0.36 | 22 |
| In vivo | 18.80 | 0.33 | 54 |
| Sex | |||
| Female | 18.27 | 0.31 | 42 |
| Male | 19.02 | 0.36 | 34 |
| Rearing history | |||
| Mother | 18.90 | 0.44 | 18 |
| Nursery | 18.81 | 0.36 | 45 |
| Wild | 18.23 | 0.47 | 13 |
Note: Values are summed GI values across all 10 slices. For average GI values, divide the summed values by 10. SE, standard error.
Anterior-Posterior Gyrification Patterns
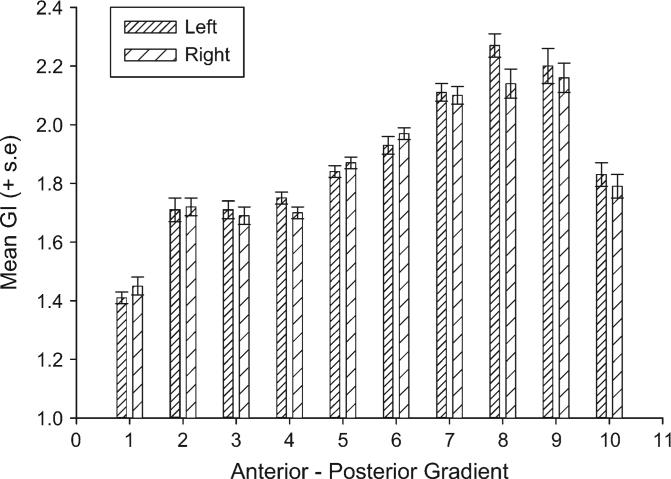
For this analysis, a mixed model analysis of variance was performed with hemisphere (left, right) and slice (1−10) serving as repeated measures. Sex (male, female) and rearing history (mother, nursery, wild) were the between-group factors. The dependent variable was the GI score. A significant main effect for brain slice (F9,531 = 86.50, P < 0.004) was found as well as a significant 2-way interaction between hemisphere and brain slice (F9,630 = 2.33, P < 0.02). The mean GI scores for the left and right hemispheres for each section are shown in Figure 3. Regarding the slice difference, the mean GI scores for slice 1 were significantly lower than all other slices. Slices 2, 3, and 4 did not differ significantly from each other but were significantly lower than slices 5−9 but not slice 10. Slices 5 and 6 differed significantly from all other slices. Slices 7, 8, and 9 did not differ significantly from each other but differed from all other slices. Lastly, slice 10 differed significantly from all slices with the exception of slices 2, 3, and 4, respectively. For the laterality effects, there were significant leftward asymmetries for slices 4, 8, 9, and 10 and significant rightward biases for slices 1 and 6.
Figure 3.
Mean GI scores for each of the 10 anterior-to-posterior sections in the left and right cerebral hemispheres.
Handedness Effects on Asymmetries in Gyrification
Initially we compared the SUM-AQGI scores for each of the 4 handedness measures. Subsequently, we compared the asymmetry quotient (AQ) scores for each slice as a function of handedness measure to assess whether any overall effects were localized to specific regions of the brain. With respect to the SUM-AQGI scores, significant differences were found between right- and nonright-handed chimpanzees for throwing (t29 = 2.29, P < 0.02) but not for manual gestures, the TUBE or REACH tasks. The mean SUM-AQGI scores for right- and nonright-handed chimpanzees for each measure are shown in Table 2.
Table 2.
Mean gyrification AQ values for right- and nonright-handed chimpanzees for each behavioral measure
| Handedness measure | ||||||||
|---|---|---|---|---|---|---|---|---|
| Handedness | THROW | GESTURE | TUBE | REACH | ||||
| NR | R | NR | R | NR | R | NR | R | |
| N | 10 | 21 | 31 | 37 | 31 | 43 | 45 | 25 |
| Slice | ||||||||
| 1 | 0.076 | 0.035 | 0.034 | 0.039 | 0.028 | 0.033 | 0.038 | 0.038 |
| 2 | 0.027 | −0.026 | −0.005 | −0.003 | −0.021 | 0.018 | −0.017 | −0.006 |
| 3 | 0.021 | −0.040 | −0.023 | −0.013 | −0.027 | −0.003 | −0.013 | −0.011 |
| 4 | 0.006 | −0.076 | −0.009 | −0.056 | −0.032 | −0.025 | −0.037 | −0.032 |
| 5 | 0.067 | 0.003 | 0.033 | 0.012 | 0.031 | 0.013 | 0.016 | 0.031 |
| 6 | 0.067 | 0.001 | 0.039 | 0.006 | 0.023 | 0.022 | 0.017 | 0.037 |
| 7 | 0.010 | 0.008 | 0.007 | −0.010 | −0.027 | 0.008 | 0.005 | −0.030 |
| 8 | 0.046 | −0.078 | −0.059 | −0.057 | −0.060 | −0.040 | −0.055 | −0.043 |
| 9 | 0.005 | −0.028 | −0.044 | −0.029 | −0.029 | −0.025 | −0.036 | −0.036 |
| 10 | −0.082 | 0.028 | −0.029 | −0.047 | −0.045 | −0.033 | −0.054 | −0.017 |
Note: Bolded values indicate significant differences between nonright- (NR) and right-handed (R) subjects. Sample sizes for each group are indicated under the designation of handedness. Slices 1−10 follow an anterior-to-posterior gradient.
For the region specific analyses, Table 2 lists the mean AQ scores by slice and handedness measure. For throwing, significant differences in the AQ scores were found between right- and nonright-handed chimpanzees for slice 4 (t29 = 2.07, P < 0.05), slice 6 (t29 = 2.28, P < 0.04), and slice 8 (t29 = 2.57, P < 0.01). For all 3 slices, right-handed chimpanzees had lower AQ scores (indicative of greater left hemisphere gyrification) compared with nonright-handed chimpanzees. For manual gesture, significant differences were found for slice 4 (t60 = 1.98, P < 0.05). As with the throwing measure, right-handed chimpanzees had lower AQ scores (indicative of greater left hemisphere gyrification) compared with nonright-handed chimpanzees. No significant differences were found for any slice in right- and nonright-handed chimpanzees for the TUBE and REACH measures.
Age and Brain Volume Correlates of Gyrification
A series of Pearson product moment and partial correlation coefficients were performed to evaluate the associations between age, brain volume, and the various indices of gyrification. Neither brain volume nor body weight significantly correlated with the SUM-GI scores for the sample. To evaluate the influence of age on gyrification, a partial correlation coefficient was performed, adjusting for specimen type (cadaver, in vivo) and brain volume. After adjusting alpha for multiple tests, this analysis revealed a significant positive association between age and the GI1 measure (r = 0.343, degrees of freedom = 73, P < 0.005) (see Table 3). Older subjects had larger GI values for the most anterior slice of the cortex. None of the remaining correlation coefficients reach conventional levels of statistical significance.
Table 3.
Partial correlation coefficients between GI values for each slice and age
| Slice | Overall | Females | Males |
|---|---|---|---|
| 1 | 0.343* | 0.459* | 0.146 |
| 2 | 0.175 | 0.259 | 0.143 |
| 3 | −0.023 | 0.029 | −0.090 |
| 4 | −0.185 | −0.253 | −0.118 |
| 5 | −0.047 | −0.119 | 0.097 |
| 6 | −0.129 | −0.210 | 0.027 |
| 7 | −0.125 | −0.213 | −0.059 |
| 8 | −0.175 | −0.325 | 0.113 |
| 9 | −0.092 | −0.139 | 0.000 |
| 10 | −0.115 | −0.257 | 0.026 |
| Overall | −0.080 | −0.141 | −0.045 |
Note:
indicates P < 0.005. Slices 1−10 follow an anterior-to-posterior gradient.
Discussion
Four significant findings were revealed in this study. First, chimpanzees show regional variation in gyrification of the brain, and these results are consistent with previous findings in other primates, although with much smaller sample sizes. Second, chimpanzees show a population-level leftward asymmetry in gyrification. We believe these are the first evidence demonstrating population-level asymmetries in gyrification in a primate species. Third, handedness influences gyrification with right-handed subjects showing greater gyrification in the left compared with right hemisphere. In contrast, nonright-handed subjects show no differences in gyrification between the left and right hemispheres. Lastly, the sensitivity of the behavioral measure of handedness in detecting population-level handedness influences the manifestation of gyrification asymmetry seen in the brain. Behavioral measures that induce greater preferential use of the right hand show greater differentiation in gyrification asymmetries between hemispheres.
Regarding the general pattern of gyrification seen in our study, the findings indicated smaller GI scores at the frontal and occipital poles but, in general, a rostral to caudal increasing gradient in gyrification. This pattern of results is consistent with previous studies in primates using cadaver specimens (Zilles et al. 1989) and MRI (Rilling and Insel 1999b). It should be emphasized that the GI scores for each slice were quite consistent in our sample between scanned cadaver brains and in vivo scans, suggesting that the different scanning protocols yielded similar resolution in sulcal patterns, and this is consistent with previous results in primates (see Rilling and Insel 1999b). Our overall GI scores were lower than previous values reported for chimpanzees (Zilles et al. 1989; Rilling and Insel 1999b), but this may reflect differences in the sample sizes deriving the different data sets. In fact, the GI values obtained in this sample for 5 chimpanzees that were part of the study by Rilling and Insel (1999b) were quite comparable (GI = 2.09 and 2.19, respectively); thus, the overall lower values obtained in this sample more likely approximate the values for chimpanzees.
There were neither significant sex nor rearing effects on overall GI or regional variation in GI scores as well as asymmetries in gyrification. For the most part, these findings are consistent with previous reports in humans and primates, save the recent findings by Luders et al. (2004). Luders et al. (2004) found greater gyrification in females, but the method of analysis and quantification of gyrification was different from those used in this and previous studies. The different methods may contribute to the lack of consistent results between studies.
With respect to asymmetries, the chimpanzees showed regional variation in patterns of asymmetry with the frontal pole (AQ1) and slice 6 showing rightward asymmetries, whereas leftward biases were evidenced in the posterior frontal (slices 4 and 5) and temporal-occipital brain slices (slices 8 and 10), findings consistent with those on asymmetry in sulci complexity in humans (Luders et al. 2004). In many ways, the patterns of asymmetry in gyrification resemble the right frontal, left occipital asymmetries in cerebral torque that have been described in both human and nonhuman primates from endocasts and from direct measures of the brain (LeMay 1976; Weinberger et al. 1981; Holloway and De La Costa-Lareymondie 1982; Pilcher et al. 2001; Barrick et al. 2005). Thus, asymmetries in both cerebral torque and gyrification may reflect a common neural developmental pattern of the cortex.
Previous studies in humans (Zilles et al. 1988), nonhuman primates (Rilling and Insel 1999b), and other mammals (Wosinski et al. 1996; Mayhew et al. 1996) have not reported any evidence of asymmetries; however, at least with respect to the nonhuman primate and dog studies, samples sizes were relatively small and there was considerable variation in the species comprising specific taxonomic families. For example, in the Rilling and Insel (1999b) study on nonhuman primates, the taxonomic family of great apes was made up of 4 different species, and the sample size within a species was small. Our study consisted of only one great ape species with a much larger sample size. In light of the fact that the asymmetry effect size is small, this seems like an important difference. Evidence of asymmetries for gyrification in humans have not been consistently reported, but this is difficult because of the emphasis on comparison between control and clinical populations (also see Luders et al. 2004). Zilles et al. (1989) did test for asymmetries in their sample of 61 human subjects and reported no significant difference, but our own analysis of their raw data (which is provided in Table 1 of Zilles et al. 1989) revealed a significant difference in the left and right GI values (t60 = 2.18, P < 0.04). The mean difference between the left and right hemisphere GI values was small (mean difference = 0.021) and rivaled that which was observed in the chimpanzees (mean difference = 0.032). Thus, humans and chimpanzees show a small but significant left hemisphere asymmetry in gyrification of the cerebral cortex.
The handedness of the chimpanzees influenced gyrification values of the left and right hemispheres. For throwing, and to a lesser extent manual gestures, right-handed subjects had greater gyrification values for the left compared with the right hemisphere. Moreover, the pattern of asymmetry was more pronounced in the rostral and midslices of the cortex, notably slices 3−5, which correspond roughly to premotor, primary motor, and primary somatosensory cortex. This pattern of results was not found in subjects classified as nonright handed. These results suggest that handedness is associated with greater sulci depth and gyrification in the hemisphere contralateral to the preferred hand. These results are largely consistent with the previous findings by Hopkins and Cantalupo (2004) as well as Taglialatela et al. (2006) that reported differences in volumes of the inferior frontal gyrus and motor-hand area of the left and right hemisphere in left- and right-handed subjects and further support the view of significant structural-functional associations in the laterality of chimpanzees and perhaps other non-human primates (Phillips and Sherwood 2005). This converging evidence clearly indicates the usefulness of assessing macro-structural measures of asymmetry to discover significant structural-functional associations in the laterality of chimpanzees. The findings further highlight the observation that not all measures of hand use are sensitive to variation in neuroanatomical asymmetries, making the selection of behavioral tasks very important when trying to establish brain-behavior relationships.
Brain volume and body weight did not correlate with individual differences in gyrification in chimpanzees. These results are consistent with previous findings in adult humans (Zilles et al. 1989) and rhesus monkeys (Armstrong et al. 1995) but not dogs (Wosinski et al. 1996). Clearly from a phylogenetic standpoint, brain size influences gyrification, but within species of primates, individual differences do not appear to be explained solely by brain size. Why these results differ from findings in dogs is not clear, but there were mixed species of canines in the study of Wosinski et al. and if the analyses were restricted to single species of canine, perhaps no association between brain size and gyrification would be found as well.
A significant positive association was found between age and the GI1 values, and this association was more pronounced for the female compared with male chimpanzees.
Previous studies in humans have reported increasing gyrification with age, and this largely reflects greater atrophy of the brain that results in increasing widths and depths of the sulci (Magnotta et al. 1999; Kochunov et al. 2005). Chimpanzees do show loss in brain volume with increasing age (Herndon et al. 1999), suggesting neural loss that might explain the increasing gyrification in older chimpanzees. Whether there is selective neuronal loss in aged chimpanzee brains is unknown, but these data would add important information for interpreting the observed association between GI1 and age in our sample. As noted above, the results were more pronounced for females compared with males, and the reason for this difference is unclear. The age range of females (5−45 years) and males (10−44 years) was comparable in our sample and therefore do not appear to be confounding factors. However, there were 6 females 40 years or older in our sample, whereas only one male was more than 40 years of age. Alternatively, female chimpanzees live longer than male chimpanzees by more than 10 years by some estimates (Dyke et al. 1996). The longer life span of the female chimpanzee may increase the likelihood of observing age-related changes in neuron loss, as reflected in increased GI scores, and these effects might be selective to the prefrontal regions in the chimpanzee. Given the importance of prefrontal cortex in a variety of cognitive skills, this would result in predictions of greater cognitive loss in female compared with male chimpanzees. Unfortunately, the virtual absence of behavioral and brain imaging data from aged chimpanzees precludes any definitive conclusions from being drawn.
In conclusion, the results of this study confirm previous studies showing an anterior-to-posterior gradient in gyrification of the cerebral cortex of chimpanzees, albeit with a larger sample of apes than previous studies. The results further indicate that asymmetries in gyrification are present in chimpanzees and influenced by the handedness of the subjects. We believe these are the first evidence of an association between handedness in asymmetries in gyrification in chimpanzees. Whether similar patterns of results are evident in other species, including humans, remains unknown but should be considered in future studies. Moreover, further studies on asymmetries in gyrification of well-known morphological areas, such as Wernicke's area and inferior frontal gyrus, would be useful from the standpoint of identifying potential brain areas that have been selected upon in primate evolution for uniquely human behavioral or cognitive functions, such as speech and higher cognitive processes.
Acknowledgments
This work was supported in part by National Institutes of Health grants RR-00165, NS-42867, NS-36605, and HD-38051. We thank the care staff from each facility for assistance in data collection. The YRPRC facility is accredited by the American Association of Laboratory Animal Care. American Psychological Association guidelines for the care and use of animals were adhered to during all aspects of this study. We thank Hani Freeman and Leslie Dunham for assistance in tracing brain scans.
Footnotes
Conflict of Interest: None declared.
References
- Armstrong E, Curtis M, Buxhoeveden DP, Fregoe C, Zilles K, Casanova MF, McCarthy WF. Cortical gyrification in the rhesus monkey: a test of the mechanical folding hypothesis. Cereb Cortex. 1991;1:426–432. doi: 10.1093/cercor/1.5.426. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;1:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Zilles K, Schleicher A. Cortical folding and the evolution of the human brain. J Hum Evol. 1993;20:341–348. [Google Scholar]
- Barrick TR, Mackay CE, Prima S, Maes F, Vandermeulen D, Crow TJ, Roberts N. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24:678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Araque J, Giedd J, Rumset JM. Reduced brain size and gyrification in the brains of dyslexic patients. J Child Neurol. 2004;19:275–281. doi: 10.1177/088307380401900407. [DOI] [PubMed] [Google Scholar]
- Deacon TW. The symbolic species. W. W. Norton; New York: 1997. [Google Scholar]
- Dyke B, Williams-Blangero S, Mamelka PM, Goodwin WJ. Future costs of chimpanzees in US research institutions. Inst Lab Anim Res. 1996;37:193–198. doi: 10.1093/ilar.37.4.193. [DOI] [PubMed] [Google Scholar]
- Harris JM, Whalley H, Yates S, Miller P, Johnstone C, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol Psychiatry. 2004;56:182–189. doi: 10.1016/j.biopsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain weight throughout the life span of the chimpanzee. J Comp Neurol. 1999;409:567–572. [PubMed] [Google Scholar]
- Holloway RL, De La Costa-Lareymondie MC. Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am J Phys Anthropol. 1982;58:101–110. doi: 10.1002/ajpa.1330580111. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. J Comp Psychol. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Evolution of language and speech from a neuropsychological perspective: some recent findings in great apes and their theoretical implications. In: Washburn DA, editor. Primate perspectives on behavior and cognition. American Psychological Association; Washington (DC): 2006. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): evidence of a left hemisphere specialization in motor skill. J Exp Psychol Gen. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes). Psychol Sci. 2005;16:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Freeman H, Cantalupo C, Schapiro S. Factors influencing the prevalence and handedness for throwing in captive chimpanzee. J Comp Psychol. 2005;119:363–370. doi: 10.1037/0735-7036.119.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees are predominantly right-handed: replication in three colonies of apes. Behav Neurosci. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of the brain and intelligence. Academic Press; New York: 1973. [Google Scholar]
- Kochunov P, Mangin JF, Coyle T, Lancaster J, Thompson P, Riviere D, Cointepas Y, Regis J, Schlosser A, Royall DR, et al. Age-related morphology trends in cortical sulci. Hum Brain Mapp. 2005;26:210–220. doi: 10.1002/hbm.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man and nonhuman primates. Ann N Y Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, Toga AW. Gender differences in cortical complexity. Nat Neurosci. 2004;7:799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, Nopoulos P, Flaum M. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9:151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Mwamengele GLM, Dantzer V, Williams S. The gyrification of mammalian cerebral cortex: quantitative evidence of anisomorphic surface expansion during phylogenetic and ontogenetic development. J Anat. 1996;188:53–58. [PMC free article] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearb Phys Anthropol. 1997;40:201–232. [Google Scholar]
- Passingham R. The human primate. W. H. Freeman; San Francisco, CA: 1982. [Google Scholar]
- Phillips K, Sherwood CC. Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys (Cebus apella). Behav Neurosci. 2005;119:1701–1704. doi: 10.1037/0735-7044.119.6.1701. [DOI] [PubMed] [Google Scholar]
- Pilcher DL, Hammock EAD, Hopkins WD. Cerebral volumetric asymmetries in non-human primates: a magnetic resonance imaging study. Laterality. 2001;6:165–179. doi: 10.1080/13576500042000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T. What's human about the human brain. In: Gazzaniga MS, editor. The new cognitive neurosciences. MIT Press; Champagne, IL: 2000. pp. 1219–1234. [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rilling JR. Human and nonhuman primate brains: are they allometrically scaled versions of the same design? Evol Anthropol. 2006;15:65–77. [Google Scholar]
- Rilling JK, Insel TR. Evolution of the cerebellum in primates: differences in relative volume among monkeys, apes and humans. Brain Behav Evol. 1998;52:308–314. doi: 10.1159/000006575. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. Differential expansion of neural projection systems in primate brain evolution. Neuroreport. 1999a;10:1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999b;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Seligman RA. A quantitative morphometric comparative analysis of the primate temporal lobe. J Hum Evol. 2002;42:505–533. doi: 10.1006/jhev.2001.0537. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci. 2005;8:242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H. The brain and it major anatomical subdivisions in living hominoids using magnetic resonance imaging. J Hum Evol. 2000;38:317–332. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- Sherwood CS, Holloway RL, Semendeferi K, Hof PR. Is prefrontal white matter enlargement a human evolutionary specialization? Nat Neurosci. 2005;8:537–538. doi: 10.1038/nn0505-537. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures insectivores and primates. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Taglialatela J, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. Neuroreport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Luchins DJ, Morihisa J, Wyatt RJ. Asymmetrical volumes of the right and left frontal and occipital regions of the human brain. Ann Neurol. 1981;11:97–100. doi: 10.1002/ana.410110118. [DOI] [PubMed] [Google Scholar]
- White T, Andreason NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Wosinski M, Schleicher A, Zilles K. Quantitative analysis of gyrification of cerebral cortex of dogs. Neurobiology. 1996;4:441–468. [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Kretchmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol. 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]