Abstract
It has been suggested from studies in human subjects that sex, handedness, and brain asymmetries influence variation in corpus callosum (CC) size and these differences reflect the degree of connectivity between homotopic regions of the left and right cerebral hemispheres. Here we report that handedness is associated with variation in the size of the CC in chimpanzees. We further report that variation in brain asymmetries in a cortical region homologous to Broca's area is associated with the size of the CC but differs for right- and left-handed individuals. Collectively, the results suggest that individual differences in functional and neuroanatomical asymmetries are associated with CC variation not just in humans but also in chimpanzees and therefore may reflect a common neural basis for laterality in these 2 species.
Keywords: brain asymmetry, chimpanzees, corpus callosum, handedness
Introduction
The corpus callosum (CC) is the major tract of fibers connecting homotopic cortical regions of the left and right cerebral hemispheres. The role of the CC in interhemispheric transfer of information and duality of cognitive functions has been demonstrated extensively in studies of split-brain patients and animals (Gazzaniga 2000) as well as individuals with agenesis of the CC (Jancke et al. 1997b). It has been hypothesized that variation in the size of the CC can explain both individual and evolutionary differences in behavioral and brain asymmetries (Ringo et al. 1994; Rilling and Insel 1999; Aboitiz et al. 2003). Specifically, it has been suggested that as brain size increases, interhemispheric connectivity decreases, resulting in greater neuroanatomical and behavioral specializations of the left and right cerebral hemispheres, and increased intrahemispheric connectivity. From a phylogenetic perspective, there is some support for this theory. Recent comparative studies in vertebrates, including nonhuman primates, have shown an inverse relationship between brain size and CC morphology as well as fiber density (Rilling and Insel 1999; Olivares et al. 2000, 2001). Moreover, in primates, it has also been reported that species with smaller CC to brain size ratios have larger brain asymmetries (Hopkins and Rilling 2000) than species with larger ratios.
Less clear from the literature is whether or not individual differences in brain size and asymmetries are associated with CC morphology (see Jancke et al. 1997a). In humans, a significant body of research has accumulated examining CC size differences between sexes and handedness groups (Witelson 1985; Habib et al. 1991; Burke and Yeo 1994; Driesen and Raz 1995; Bishop and Wahlsten 1997). In general, the results indicate that males have smaller CCs than females, and that left-handed or inconsistently handed subjects have larger CCs compared with right- or consistently handed individuals (i.e., those with either a left or right hand preference). Notwithstanding, the findings have not always been consistent among studies and differences may reflect 1) the types of specimens used (post mortem vs. in vivo imaging), 2) the manner in which the CC is quantified, and 3) with respect to handedness, the procedure by which subjects are classified as left- or right-handed.
Less studied in humans has been the association between neuroanatomical asymmetries and CC size, particularly in relation to handedness or other functional asymmetries (Clarke et al. 1993; Clarke and Zaidel 1994; Moffatt et al. 1997; Dorion et al. 2000). Some have reported negative associations between magnitudes of brain asymmetry in the posterior sylvian fissure and CC size, particularly in males (Aboitiz et al. 1996). Luders et al. (2003), in one of the only studies to examine the modulating effect of handedness on the association between brain asymmetries and CC size, found a significant association between sylvian fissure length and total and anterior CC size, particularly among right-handed subjects. In contrast, the splenium positively correlated with asymmetries in the post-central sulcus. Clearly more studies are needed in which the combined effect of handedness and brain asymmetry in relation to CC size is assessed because handedness may not necessarily be the best indicator of all neuroanatomical asymmetries. For example, correlations between handedness and asymmetries in the planum temporale (PT), inferior frontal lobe, and motor-hand area, although significant, seldom account for more than 50% of the variance (Foundas et al. 1995; Beaton 1997; Hammond 2002). Thus, there is variability in directional biases in brain asymmetries within defined handedness groups and therefore the combined rather than the independent effects of handedness and brain asymmetries might potentially explain a greater amount of variability in CC size.
When considering data from nonhuman animals, there are almost no studies on the relation between behavioral or brain asymmetries and CC size. It has been reported that the size of the CC in male rats is larger than in females and these effects can be modified by early handling experiences as well as prenatal hormone exposure (Denenberg et al. 1991). Furthermore, rats with more asymmetric brains have smaller CCs compared with rats with less asymmetric brains (summarized in Rosen 1996). Several studies on mice with callosal agenesis or surgical transection of the CC have shown that they are significantly left-pawed and the incidence of complete agenesis is greater in females compared with males (Manhães et al. 2002, 2003, 2005). In dogs, the CC is larger in right- compared with left-pawed individuals (Aydinlioğlu et al. 2000), a finding similar to those recently reported by Dunham and Hopkins (in press) in chimpanzees. Franklin et al. (2000) did find that female rhesus monkeys have smaller CC compared with males, however, no correction was made for differences in brain volume.
The paucity of data from nonhuman animals on limb preferences and brain asymmetry in relation to CC size is problematic given the assumption that associations between CC size and brain asymmetry underlie the organization of lateralization in the central nervous system of all vertebrates (see Galaburda et al. 1990; Rosen 1996). The complete absence of data from chimpanzees is particularly unfortunate because recent studies have shown that great apes, like humans, exhibit population-level handedness for a variety of tasks (Hopkins in press) as well as leftward neuroanatomical asymmetries in brain regions homologous to the human language areas including Broca's and Werrnicke's areas (Gannon et al. 1998; Hopkins et al. 1998; Cantalupo and Hopkins 2001; Cantalupo et al. 2003). In addition, there is some evidence that variation in hand preferences is associated with neuroanatomical asymmetries in the primary motor cortex as well as regions of the inferior frontal gyrus (IFG) (Hopkins and Cantalupo 2004; Taglialatela et al. 2006).
The purpose of this study was to examine whether or not handedness modulates the association between brain asymmetries and CC size in a sample of chimpanzees. Based on behavioral and brain asymmetries identified previously in chimpanzees, it was hypothesized that individual differences in CC morphology would be associated with variation in laterality within this species. Specifically, our hypothesis was that if laterality is associated with a reduction in interhemispheric connectivity in chimpanzees, as manifest by smaller CC, then more lateralized subjects should show significantly smaller CC than less lateralized apes. Moreover, if handedness represents a functional asymmetry in chimpanzees, then it was further hypothesized that variation in the association between CC size and brain asymmetries would differ between left- and right-handed individuals.
Method
Subjects
Behavioral assessment of handedness and magnetic resonance images (MRIs) were collected in a sample of 60 captive chimpanzees (Pan troglodytes) including 32 females and 28 males ranging in age from 6 to 45 years (mean = 22.06, SD = 11.39). All the chimpanzees are or were members of a captive colony housed at Yerkes National Primate Research Center (YNPRC) in Atlanta, GA. Twelve of the brains were scanned post mortem, whereas the other 48 subjects were alive and healthy at the time of the scan.
Image Collection and Procedure
MRIs were obtained from cadaver specimens and in vivo. The cadaver specimens (n = 12) were stored in a solution of water and 10% formaldehyde for intervals ranging from 1 week to 5 years and were scanned with a 4.7-T magnet (Bruker, BioSpec). For the in vivo scans, subjects were first immobilized by ketamine injection (10 mg/kg) and subsequently anaesthetized with propofol (40−60 mg/kg/h) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility. The subjects remained anaesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time ∼2 h). Subjects were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 80 min as a function of brain size. The majority of the subjects (n = 38) were scanned using a 1.5-T scanner (Phillips, Model 51). The remaining chimpanzees (n = 10) were scanned using a 3.0-T scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, PA) at the YNPRC.
For all chimpanzees scanned in vivo using the 1.5-T machine, T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged 8, and a 256 × 256 matrix). For the 12 postmortem scans, T2-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 22.0 s, echo time = 78.0 ms, number of signals averaged = 8−12, and a 256 × 192 matrix reconstructed to 256 × 256). Ten of the chimpanzees were scanned using a 3.0-T scanner (Siemens Trio). T1-weighted images were collected using a 3-dimensional gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320).
After completing MRI procedures, the subjects scanned in vivo were returned to the YNPRC and temporarily housed in a single cage for 6−12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage. The archived MRI data were transferred to a PC running Analyze 6.0 (Mayo Clinic, Mayo Foundation, Rochester, MN) software for postimage processing.
Brain Regions of Interest
CC Measurement
CC area measurements were taken from the midsagittal slice using a method similar to that described by Witelson (1989). The method divides the CC into 7 segments, which are roughly associated with different sets of fiber projections to various cortical regions of the brain (LaMantia and Rakic 1990; Pandya et al. 1986; Witelson 1989). Witelson's (1989) study was conducted on post-mortem brain tissue, therefore the method was adapted for use with MRI scans and computer analysis (see Fig. 1). Using the region of interest function (ROI) within ANALYZE, the CC was first divided into thirds. The anterior third was then subdivided into 3 regions by inserting a vertical line through the point where the anterior CC begins to curve back slightly. This delineated regions 1, 2, and 3, which are congruent to Witelson's (1989) rostrum, genu, and rostrum body. The region of the middle third was subdivided into 2 equal sections, creating regions 4 and 5 referred to as the anterior and posterior midbodies. The posterior third was further subdivided into 2 sections, the isthmus and splenium. Witelson (1989) defined the splenium as occupying the posterior one-fifth of the total CC length. To delineate this region, a total CC length measurement, from the most anterior point to the most posterior point of the CC, was taken. Based on the length measurement, a vertical line was drawn through the CC to define the posterior one-fifth as the splenium. The remaining section, just anterior to the splenium, was congruent to Witelson's (1989) isthmus. Using the tracing tool, the area (in mm2) of the CC lying within each outlined region was measured in each individual.
Figure 1.
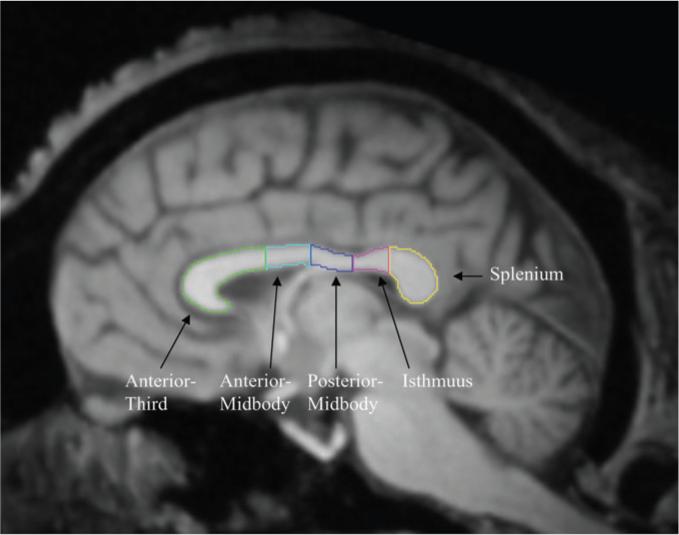
Midsagittal slice of a chimpanzee brain with each measured region of the CC outlined according to the procedure used by Luders et al. (2003).
Consistent with previous studies (Luders et al. 2003), we combined the area measures for the 3 most anterior regions (rostrum, genu, and rostrum-midbody) and referred to this as the anterior CC region. Areas measures of the CC for each region were calculated for each subject. Brain volumes were also obtained using an automated segmenting module within ANALYZE and included white and gray matter as well as the ventricles but not the cerebellum or brain stem structures. L.D. traced all the CC and was blind to the sex and handedness of the subjects. To assess reliability, 2 individuals (L.D. and J.T.) blind to the handedness of the subjects independently measured the entire CC and each region within the CC in a sample of 10 chimpanzees. For the entire CC, the correlation was positive and significant (r = 0.81, df = 8, P < 0.01). The correlation coefficients for each region of the CC ranged from 0.69 to 0.89, all of which were significant at P < 0.05.
Brain Asymmetry Measurements
Two brains regions considered homologous to the classic language areas of the human brain were measured in this study including the frontal-orbital sulcus (FO) and PT. Population-level leftward asymmetries have been reported for both of these regions in chimpanzees (Cantalupo and Hopkins 2001; Cantalupo et al. 2003; Hopkins and Cantalupo 2004) and therefore they were ideal for characterizing the association between brain asymmetry and handedness. The procedure used to trace reach region is described below. All experimenters tracing the MRI scans were blind to the sex and handedness of the subjects. Two individuals blind to the handedness of the subjects and orientation of the brain independently traced FO and PT. Interrater correlation coefficients between 2 individuals were positive and significant for FO (r = 0.998, df = 6, P < 0.001) and PT (r = 0.947, df = 6, P < 0.01).
FO sulcus
A portion of the IFG, where part of Broca's area is located, was measured by tracing the length of the FO sulcus, a prominent landmark of the opercular portion of the IFG (see von Bonin 1949; Cantalupo and Hopkins 2001; Sherwood et al. 2003). FO could be clearly seen in para-sagittal (1 mm thick) MRI slices, and its length was traced from the first lateral slice where it was present up to the slice immediately preceding the opening of the insula (between 4 and 9 slices). The lengths of FO within each hemisphere were summed across slices to derive an estimate of the area of this region.
Planum temporale
To measure the surface area of PT, the MRI scans were aligned in the coronal planes and cut into 1-mm slices using multiplanar reformatting software (ANALYZE). Coronal rather than sagittal image sequences were used because, according to some, these provide the best direct assessment of the full depth of the sylvian fossa of which the PT is its floor (Larsen et al. 1989; Shapleske et al. 1999). The anterior border of the PT was defined by the most rostral slice showing Heschl's gyrus (HG). The posterior border was defined by the most caudal slice showing the sylvian fissure. Once the anterior and posterior borders were delineated, the depth of SF (i.e., width of the PT) on each slice was measured from the superolateral margin of the superior temporal gyrus. Depth measures were taken up to the lateral ridge of HG in all the slices where HG was present (normally, HG was no longer present in slices proximal to the posterior border of PT). Following a well-established procedure in the human literature, an estimate of the PT surface areas (in mm2) was computed as the sum of the cumulative PT depth measures for each slice within a hemisphere multiplied by the slice thickness.
Handedness Measurement
Like the previous study by Dunham and Hopkins (in press) in chimpanzees, a composite measure of handedness was derived from 4 measures of hand use previously described in these subjects including manual gestures (Hopkins et al. 2005), simple reaching (Hopkins et al. 2002), bimanual feeding (Hopkins 1994), and a task measuring coordinated bimanual actions, referred to as the TUBE task (Hopkins 1995). These 4 measures were selected on which to derive a single measure of handedness because 1) they were uncorrelated with each other (see Hopkins in press), 2) they each elicit consistent hand preferences in the chimpanzees, and 3) these measures were available in the largest cohort of subjects. A brief description of each measure is provided below.
Manual Gestures
At the onset of each trial, an experimenter would approach the chimpanzee's home cage and center themselves in front of the chimpanzee at a distance of approximately 1.0−1.5 m. If the chimpanzee was not already positioned in front of the experimenter at the onset of the trial, the chimpanzee would immediately move toward the front of the cage when the experimenter arrived with the food. The experimenter then called the chimpanzee's name and offered a piece of food until the chimpanzee produced a manual gesture. Only responses in which the chimpanzee's unimanually extended their digit(s) through the cage mesh to request the food were considered a response. Other possible manual responses such as cage banging or clapping were not counted as a gesture. Two-handed gestures, although rare, were not scored as were gestures that were produced by the chimpanzee prior to the experimenter arriving in front of the chimpanzee's home cage. When the chimpanzees produced a unimanual gesture, the experimenter recorded hand use as left or right.
Simple Reaching
On each trial, a raisin was thrown into the subject's home cage. The raisin was thrown by the experimenter to a location at least 3 m from the focal subject so that the chimpanzees had to locomote to position to the raisin, pick up the raisin, and bring it to their mouth for consumption. When the chimpanzee acquired the raisin, the experimenter recorded the hand used as left or right. One, and only one, reaching response was recorded each trial to assure independence of data points (see McGrew and Marchant 1997; Hopkins 1999 for contrasting views). Thus, raisins were not randomly scattered in home cages but rather an individual raisin was thrown into cages and subjects retrieved the raisin before another was thrown into the cage. Subjects were required to locomote at least 3 strides between reaching responses to maintain postural readjustment between trials.
Bimanual Feeding (FEED)
Each afternoon, the primates housed at the YNPRC receive fruits and vegetables as part of their daily diet. Each subject usually receives 2 oranges, 1 banana, some celery stalks, and/or carrots. Upon retrieving the food, the subjects typically move to a seating place and consume the food. The chimpanzees generally hold the extra pieces of food with one hand and feed with the opposite hand. Hand use was recorded when the subjects were feeding with one hand for a minimum duration of 3 seconds and the nonfeeding hand was holding the remaining portions of food. The dominant hand was recorded as the one feeding.
Coordinated Bimanual Actions (TUBE)
The second handedness measure was a task requiring bimanual coordinated actions, referred to as the TUBE task (Hopkins 1995). For the TUBE task, peanut butter is smeared on the inside edges of polyvinyl chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths but rather must use one hand to hold the tube and the other hand to remove the substrate. The PVC tubes were handed to the subjects in their home cages and a focal sampling technique was used to collect individual data from each subject. The hand of the finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter and brought it to their mouth, the hand used was recorded as left or right.
All the chimpanzees were tested in the outdoor portion of their home cages. The number of responses obtained from each subject differed within and between tasks. Notwithstanding, a minimum of 30 responses were obtained for each individual for each task. Individuals recording the hand use data were blind to the brain asymmetries of the subjects, the size of the CC, and the hypothesis of the study.
Data Analysis
Corpus Callosum
The best way to analyze differences in CC morphology is a matter of some debate (Bermudez and Zatorre 2001; Smith 2005). The principle issue is the confounding relationship between CC size and overall brain volume. To address this issue, brain volume was regressed on the total CC area as well as the area measures for each of the 5 CC regions. The individual unstandardized residuals were saved from these analyses and served as subsequent dependent variables in evaluating the influence of hand and sex on CC morphology. The residuals represent the degree of deviation in size of the CC area of each individual as predicted from their brain size. Thus, positive values represent individuals that have larger CC areas for an organism of their brain volume, whereas negative values represent individuals that have smaller CC areas for their individual brain volume. We opted to use the residual values because they have been used in previous studies on the association between handedness and CC size in chimpanzees (Dunham and Hopkins in press) as well as in comparative studies of the relative size of the CC between different primate species (Hopkins and Rilling 2000).
To be assured that the residual measures were comparable with other measures, we calculated ratio measures for the entire CC and each region by dividing the area measures by the total brain volume. The resulting ratios were then correlated with the residual CC values for the entire CC as well as the anterior region, anterior-midbody, posterior-midbody, isthmus, and splenium. The resulting correlation coefficients were 0.650, 0.755, 0.688, 0.684, 0.801, and 0.789, respectively. Each of these correlation coefficients was significant at P < 0.01.
Brain Asymmetries
For both the FO sulcus and PT, asymmetry quotients (AQ) were derived following a commonly used formula AQ = [(R – L)/R + L) × 0.5] in which positive values reflect rightward asymmetries and negative values reflected leftward biases. Based on the sign of the AQ values, we classified any chimpanzees having a negative AQ value as being left hemisphere biased and any subjects with a positive AQ as right hemisphere biased.
Hand Preference Classification
For each measure, a handedness index (HI) was determined following the formula HI = (R – L)/R + L), where R and L reflect the frequency of left and right hand use. HI values varied from –1.0 to 1.0 with negative values reflecting left hand biases and positive values reflecting right hand biases. Rather than consider each handedness measure separately in the analyses, we derived a single composite handedness value by averaging the HI values for the 4 tasks. Based on the sign of the averaged HI values, we classified subjects as right (HI > 0) or left handed (HI ≤ 0). Using this criterion, there were 39 right-handed and 21 left-handed chimpanzees in our sample.
Results
Descriptive Statistics
Few studies have examined different aspects of CC size in chimpanzees. Therefore, for the sake of providing descriptive data for this species, we have provided the mean area for the overall CC as well as the specific ROIs for males and females in our sample (see Table 1). In rhesus monkeys and rats, early rearing experiences have been shown to influence CC size (Denenberg et al. 1991; Sánchez et al. 1998). Thus, to assess whether rearing had a significant effect on CC size in our subjects, a multiple analysis of variance (MANOVA) was performed with the raw CC area measures serving as the dependent variables. Sex and rearing history (mother, nursery, wild) served as between group variables. The MANOVA was performed with and without brain volume serving as a covariate. For both analyses, no significant main effects or interactions were found. Thus, neither rearing history nor sex had a significant influence on the raw or adjusted CC size. We also examined the potential effect of sex and rearing history on the AQ values for FO and PT as well as the average handedness index. No significant main effects or interactions were found. Lastly, one sample t-tests on the AQ values for the PT, t(59) = –5.19, P < 0.001, and FO, t(59) = 2.34, P < 0.01, revealed significant population-level leftward asymmetries, whereas a population-level rightward bias was found for the average HI value, t(59) = 3.11, P < 0.01. These findings are consistent with previous reports in our laboratory (Cantalupo and Hopkins 2001; Cantalupo et al. 2003; Hopkins in press).
Table 1.
Descriptive statistics for corpus area measures and brain volume
| Females | Males | |
|---|---|---|
| Total CC mm2 | 309.33 (6.35) | 304.61 (8.36) |
| Anterior third mm2 | 125.59 (3.12) | 125.41 (4.20) |
| Anterior-midbody mm2 | 39.25 (1.21) | 38.44 (1.63) |
| Posterior-midbody mm2 | 33.47 (0.85) | 31.61 (1.14) |
| Isthmus mm2 | 28.78 (1.18) | 29.95 (1.59) |
| Splenium mm2 | 84.53 (2.29) | 80.17 (3.08) |
| Brain volume cc | 353.77 (6.95) | 386.13 (9.35) |
Values in parentheses indicate standard errors.
Handedness and Sex Differences in Brain Asymmetry
We next examined the influence of sex and handedness on brain asymmetries. For this analysis, the AQ values for the PT and FO served as repeated measures in a mixed model analysis of variance. Sex (male, female) and handedness (left, right) served as between-group factors. No significant main effects or interaction were found. The mean AQ values for left- and right-handed males and females are shown in Table 2.
Table 2.
Mean AQ values for FO in left- and right-handed male and female chimpanzees
| Left handed | Right handed | |
|---|---|---|
| Raw AQ | ||
| Males | −0.021 | −0.131 |
| SE | (0.06) | (0.05) |
| Females | −0.202 | −0.101 |
| SE | (0.07) | (0.05) |
| AQ adjusted for total CC size | ||
| Males | 0.005 | −0.150 |
| SE | (0.06) | (0.05) |
| Females | −0.193 | −0.106 |
| SE | (0.07) | (0.05) |
Because we were interested in the potential modulating effect of CC size in relation to handedness and brain asymmetry, we reran the same analysis as described above but included the overall CC residual as a covariate. The subsequent results indicated a significant 2-way interaction between handedness and brain asymmetry, F1,52 = 4.08, P < 0.04 (see Table 2). Post hoc analysis indicated that right-handed males were more leftward in their AQ values compared with left-handed males. In contrast, no significant difference was found between left- and right-handed females.
Handedness, Brain Asymmetry and Residual CC Size (ADD FOOTNOTE)
For this analysis, the total and regional residual CC values served as dependent variables in a MANOVA. Handedness (left, right), brain asymmetry (left, right), and sex (male, female) served as between-group factors. Separate analyses were performed for the FO and PT measures of brain asymmetry. For FO, the MANOVA revealed a significant 2-way interaction between handedness and brain asymmetry, F6,47 = 2.45, P < 0.04. The subsequent univariate F-tests revealed significant 2-way interactions between handedness and brain asymmetry for the total CC F1,52 = 11.92, P < 0.01, anterior F1,52 = 6.43, P < 0.02, anterior-midbody F1,52 = 12.48, P < 0.01, posterior-midbody F1,52 = 3.88, P < 0.05, and isthmus F1,52 = 4.23, P < 0.05. The mean residual CC value for the total CC and each region is shown in Figure 2. Post hoc analysis was performed using Tukey's honestly significant difference test. Despite relative large differences in the residual CC values, among left-handed chimpanzees, post hoc analyses indicated no significant differences between chimpanzees considered left or right hemisphere biased based on the sign of their AQ value. In contrast, among right-handed chimpanzees, individuals who had positive AQ values for FO had significantly higher residual CC values than individuals with negative AQ values. Moreover, among individuals with positive AQ values for FO, left-handed chimpanzees had significantly lower residual values for each CC region compared with right-handed chimpanzees. For the PT, the same analysis was performed and no significant main effects or interactions were found. Thus, there was no justification to consider the univariate F-tests for each individual CC region. For both regions, no significant main effects or interactions were found for the sex of the subjects.
Figure 2.
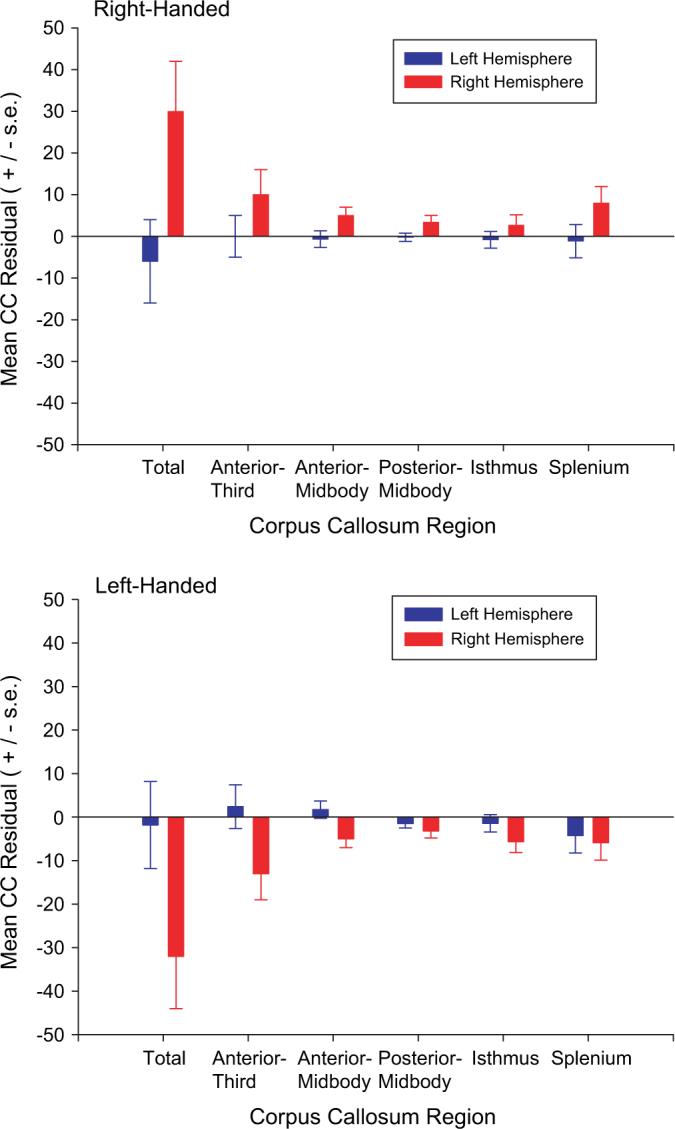
Mean residual values for the total CC and each region of the CC for left- and right-handed chimpanzees. Left hemisphere (in blue) indicates those subjects that had negative AQ values for FO, whereas right hemisphere (in red) indicates those individuals with AQ values for FO that were positive.
Correlations between AQ Measures, Handedness, and Sex
In the previous analyses, brain asymmetries were considered as a categorical variable (leftward or rightward) based on the sign of the AQ values. Because AQ values lie on a continuous scale of measurement, we used an alternative approach to assessing the association between brain asymmetry and CC size within each handedness group. Specifically, in the next analyses, the FO and PT AQ values were correlated with the residual CC values and separate correlation coefficients were performed for left- and right-handed chimpanzees as well as between males and females (see Table 3). For right-handed chimpanzees, significant associations were found between the FO AQ values and the entire CC, anterior-midbody, posterior-midbody, and isthmus. For left-handed chimpanzees, none of the correlations reached conventional levels of statistical significance. For the PT, none of the correlations were significant. For FO, a comparison between the correlation coefficients of left- and right-handed chimpanzees revealed significant differences for the overall CC as well as the anterior-midbody, posterior-midbody, and isthmus (see Table 3). Thus, right- and left-handed chimpanzees showed significant opposite associations between asymmetries in FO and regions of the CC. Among right-handed chimpanzees, more rightward lateralized subjects for FO had larger CC values. In contrast, in left-handed chimpanzees, more rightward FO values were associated with smaller CC values.
Table 3.
Correlation coefficents between FO and PT AQ values and CC size for each sex and handedness group
| CC region | Handedness | z | Sex | z | ||
|---|---|---|---|---|---|---|
| Left | Right | Males | Females | |||
| FO | ||||||
| Total | −0.306 | 0.525 | 3.11+ | 0.189 | 0.086 | 0.38 |
| Anterior third | 0.245 | 1.73* | 0.207 | −0.202 | 1.52 | |
| Anterior-midbody | −0.384 | 0.334 | 2.61+ | 0.132 | −0.143 | 1.01 |
| Posterior midbody | 0.422 | 2.33+ | 0.107 | 0.147 | 0.15 | |
| Isthmus | −0.079 | 0.489 | 2.13+ | 0.162 | 0.265 | 0.39 |
| Splenium | −0.176 | 0.281 | 1.62 | 0.049 | 0.140 | 0.18 |
| PT | ||||||
| Total | −0.319 | 0.070 | 1.78* | −0.089 | 0.095 | 0.67 |
| Anterior third | −0.003 | 1.29 | −0.143 | −0.040 | 0.38 | |
| Anterior-midbody | 0.056 | 0.14 | −0.019 | 0.161 | 0.66 | |
| Posterior midbody | −0.184 | 0.093 | 0.96 | 0.075 | 0.048 | 0.09 |
| Isthmus | −0.131 | −0.018 | 0.39 | −0.068 | −0.083 | 0.05 |
| Splenium | −0.269 | −0.062 | 0.74 | −0.091 | −0.053 | 0.14 |
Bolded values indicate significant correlation coefficients at P < 0.05. Values underlined are significant at P < 0.10. Between hand and between sex differences in correlation coefficients were statistically compared and are indicated by the corresponding z-scores. +P < 0.05,
P < 0.10.
Correlations between Absolute AQ Values, Handedness, and Sex
Rather than the direction of bias, in the next analyses we examined the association between strength of brain asymmetry, handedness, and sex. For these analyses, the absolute value of the AQ scores was calculated and used in the analyses. Separate correlation analyses between the absolute FO and PT AQ values and the CC residual values for males and females as well as left- and right-handed chimpanzees were carried out (see Table 4). Overall, there were few significant associations. With regard to hand use, among left-handed chimpanzees, more lateralized asymmetries in the PT were associated with relatively smaller CC for the total and anterior-third region. Regarding the sex of the subjects, for FO, males with larger asymmetries had smaller residual values for the total CC and isthmus.
Table 4.
Correlation coefficients between FO and PT abs-AQ values and cc size for each sex and handedness group
| CC region | Handedness | z | Sex | z | ||
|---|---|---|---|---|---|---|
| Left | Right | Males | Females | |||
| FO | ||||||
| Total | −0.087 | −0.281 | 0.69 | −0.400 | −0.044 | 1.38 |
| Anterior third | −0.003 | 0.24 | −0.326 | 0.212 | 2.03+ | |
| Anterior-midbody | −0.078 | −0.067 | 0.03 | 0.114 | 1.53 | |
| Posterior midbody | −0.135 | −0.355 | 0.37 | −0.355 | −0.226 | 0.51 |
| Isthmus | −0.242 | −0.206 | 0.13 | 0.092 | 2.44+ | |
| Splenium | −0.021 | −0.137 | 0.40 | −0.097 | −0.110 | 0.04 |
| PT | ||||||
| Total | 0.404 | −0.132 | 1.94* | 0.177 | −0.094 | 0.98 |
| Anterior third | 0.079 | 1.41 | 0.303 | 0.094 | 0.80 | |
| Anterior-midbody | 0.075 | 0.007 | 0.24 | −0.126 | 1.02 | |
| Posterior midbody | 0.215 | −0.141 | 1.25 | −0.056 | −0.004 | 0.04 |
| Isthmus | 0.201 | 0.046 | 0.54 | 0.058 | 0.091 | 0.12 |
| Splenium | 0.301 | −0.163 | 1.65* | −0.053 | 0.002 | 0.20 |
Bolded values indicate significant correlation coefficients at P < 0.05. Values underlined are significant at P < 0.10. Between hand and between sex differences in correlation coefficients were statistically compared and are indicated by the corresponding z-scores. +P < 0.05,
P < 0.10.
Discussion
Three significant findings emerged from this study. First, handedness modulates the association between CC size and brain asymmetries in the FO sulcus but not the PT in chimpanzees. Second, after adjusting for the relative size of the CC, a significant hand by sex interaction was found for the brain asymmetries. Lastly, correlational analyses between strength of brain asymmetries and relative CC size revealed some significant associations, particularly for the FO sulcus in males.
One of the main findings of this study was that the association between CC size and brain asymmetries, particularly for FO, the sulcus comprising part of the frontal operculum varies depending on the handedness of the subjects. In particular, right-handed chimpanzees that had asymmetries in FO that were ipsilateral to their preferred hand had larger CC residual values than chimpanzees that had asymmetries that were consistent with their preferred hand. To our knowledge, this is the first evidence indicating that an association between brain asymmetries and CC size is altered by their limb preference, in a nonhuman animal. It is somewhat difficult to interpret these results comparatively with findings in humans because different brain regions have been assessed between the 2 species (e.g., Luders et al. 2003). Structure-function associations in relation to CC size have largely focused on the PT in humans and our results for FO are consistent with at least some of those reports (Moffatt et al. 1997; Sequeira et al. 2006). That is, for right-handed individuals, greater rightward brain asymmetries were associated with a larger CC (Moffatt et al. 1997); however, it should be stated that the results we obtained for the PT in the chimpanzees are not entirely consistent with those in humans, in light of the fact that none of the MANOVA results reached significance. Thus, attempting to compare the findings may not be entirely legitimate given the procedural differences and kinds of tasks used to assess asymmetries between the 2 species. In point of fact, we could find no studies that have examined the association between CC size and asymmetries in the frontal operculum in human subjects.
One of the main limitations of our study with respect to the PT was the lack of subjects with a rightward asymmetry. Of the 60 subjects, 48 were classified as leftward biased (80%) and 12 were rightward biased (20%). For right-handed chimpanzees, 9 showed a rightward PT bias, whereas only 3 left-handed chimpanzees showed a rightward PT bias. Thus, there simply was not enough variability in PT asymmetry between left- and right-handed chimpanzees to make this a particularly useful comparison in our study, despite the obvious heuristic interest in this brain region. The relatively large distribution of leftward biased subjects is somewhat higher than the values reported for human brains (Beaton 1997) but is consistent with one other report of PT asymmetries in chimpanzee cadaver specimens (Gannon et al. 1998).
When one considers the correlation between the total CC size and FO for left- and right-handed chimpanzees, the pattern of results largely reflects the findings when using the MANOVA statistics. For right-handed subjects, subjects with more leftward AQ values had smaller CC sizes. In left-handed chimpanzees, the opposite pattern occurs; subjects with more leftward asymmetries had larger CC residual values. When considered collectively, the following pattern of results is evident. Left- and right-handed chimpanzees with FO asymmetries that are larger in the contralateral hemisphere have CC sizes that do not differ substantially from what would be predicted for an animal of their brain size. In contrast, right-handed chimpanzees with FO asymmetries that are larger in the ipsilateral hemisphere have large CC values for animals of their brain size. Left-handed chimpanzees with larger brain asymmetries in the hemisphere ipsilateral to their preferred hand have smaller CC values for animals of their brain size. Thus, quite different patterns in relation to FO asymmetry and CC size are found in these 2 handedness cohorts.
What explains the differential pattern of results between right- and left-handed chimpanzees in relation to FO brain asymmetries is not clear. Witelson and Nowakowski (1991) and others (Galaburda et al. 1990) have suggested that differences in CC size in right- and left-handed subjects may reflect excessive or a lack of pruning of interhemispheric cortical cells during development. These theoretical models have largely attempted to explain hand and sex difference in CC size in humans and have not necessarily considered both brain asymmetry and handedness together. Notwithstanding, if one adopts the theoretical position of Witelson and Nowakowski (1991), our results would suggest that the association between brain asymmetries and the CC in left- and right-handed chimpanzees may reflect differences in axonal pruning between hemispheres. The origin of these potential neurodevelopmental effects is not clear but post-mortem studies on CC morphology and cellular organization in chimpanzee corpus callosi would be most useful for interpreting the morphology data (see Broadfield 2001).
We found almost no evidence of sex differences in relative CC size. Specifically, for all of the MANOVA analyses, sex was a never a significant main effect nor interacted significantly with either handedness or brain asymmetry. When considering the correlation analyses, there were some significant associations found between strength of asymmetry and CC size in males but there were many correlations performed and we cannot rule out type I error as a potential explanation for this finding. Notwithstanding these limitations, it is of note that the inverse relation between brain asymmetry and CC size was in males only, a finding consistent with 2 other reports in humans (Aboitiz et al. 1996; Luders et al. 2003) and at least one report in rats (Berrebi et al. 1998). Thus, where significant associations were found, our results are consistent with the extant literature.
It could be argued that inclusion of post-mortem images may have skewed the results in someway due to shrinkage factors. If the same analyses as described above are performed only on the in vivo scans, no differences in the results are found. Thus, inclusion of the cadaver scans is appropriate and does not skew the results. Moreover, we used the residual CC values and presumably shrinkage would influence all brain regions in a similar manner. Thus, relatively speaking, the CC size as well as overall brain volume were both smaller in post mortem brains due to the same influence of the fixative agents.
It should also be noted that different approaches have been employed to statistically control for individual differences in brain size in relation to CC size (i.e., Smith 2005). We opted to use residual values but if other measures are used in our sample, comparable results are found. Specifically, if ratio measures in the size of the CC relative to brain volume are used (CC values/ brain volume) are used as the dependent measures, significant intereactions are found between handedness and brain asymmetry for the entire CC, F1,56 = 12.58, P < 0.001, as well as the anterior region, F1,56 = 9.05, P < 0.01, anterior-midbody, F1,56 = 11.68, P < 0.001, posterior-midody, F1,56 = 7.08, P < 0.01, isthmus, F1,56 = 6.89, P < 0.01, and splenium, F1,56 = 4.99, P < 0.05. For comparison to the findings using the residual values depicted in Figure 3 are the mean ratio values for left- and right-handed chimpanzees that had either positive or negative AQ values for FO (labeled left or right hemisphere biased). As can be seen, the results for the ratio values are quite comparable with the results using residual CC values. Thus, our results are consistent across different methods used in controlling for the relative difference in CC size in relation to brain volume.
Figure 3.
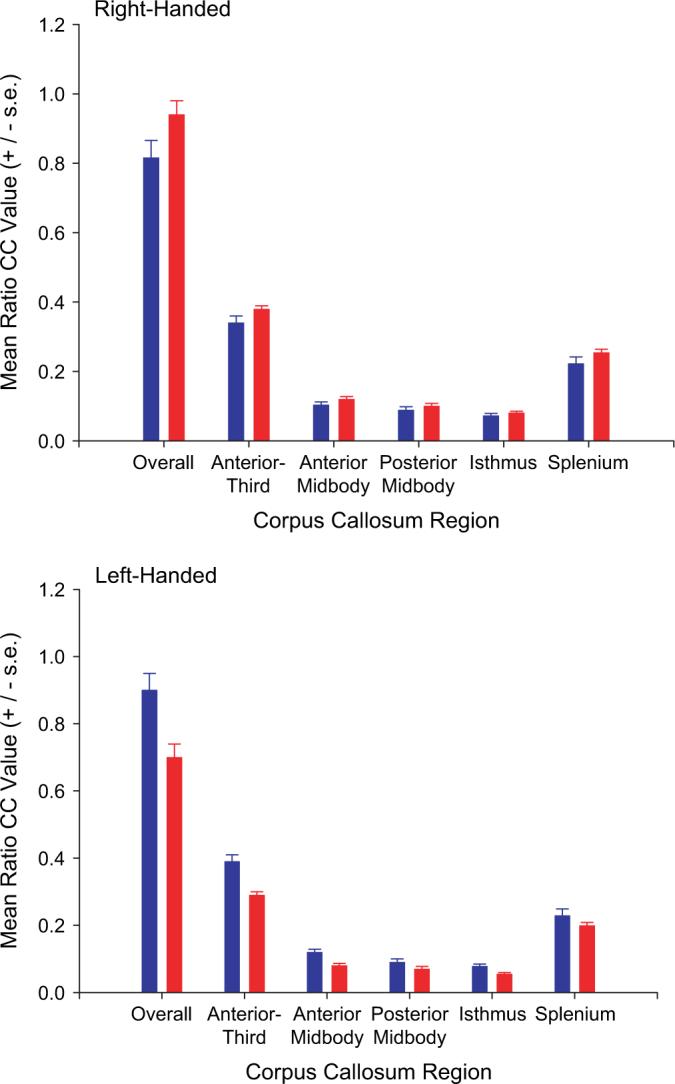
Mean ratio values for the total CC and each region of the CC for left- and right-handed chimpanzees. Left hemisphere (in blue) indicated those subjects that had negative AQ values for FO, whereas right hemisphere (in red) indicates those individuals with AQ values for FO that were positive.
In sum, the results reported here indicate that associations between the size of the CC and brain asymmetry are modulated by the handedness of the chimpanzees but not their sex. Specifically, chimpanzees with brain asymmetries in the FO sulcus consistent with their preferred hand have significantly smaller CC residual values than chimpanzees with asymmetries ipsilateral to their preferred hand and these effects are more robust for the anterior regions of the CC. Some have questioned the validity of handedness as a marker of hemispheric specialization in nonhuman primates (Warren 1980; Ettlinger 1988) and our results contradict this view. Rather, our results suggest that handedness in chimpanzees when considered alone or in conjunction with a measure of brain asymmetry explains a significant proportion of variation in CC size and therefore is a significant predictor of hemispheric specialization. Lastly, we found no compelling evidence of sex differences in the relative size of the CC in chimpanzees, a finding at odds with common lore in the human literature on CC morphology; however, reports of sex differences in CC morphology in humans are not consistent across studies and therefore should be interpreted with some caution. If sex differences in CC morphology are evident in humans and not in chimpanzees, this might represent a neurological trait unique to hominid evolution, as has been suggested by some (Holloway et al. 1993).
Notes
This work was supported in part by National Institutes of Health grants RR-00165, NS-42867, NS-36605, and HD-38051. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care, and American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study. Special thanks to the veterinary staff for assisting in the care of the animals during scanning.
Footnotes
Conflict of Interest: None declared.
References
- Aboitiz F, Lopez J, Montiel J. Long distance communication in the human brain: timing constraints for inter-hemispheric synchrony and the origin of brain lateralization. Biol Res. 2003;36:89–99. doi: 10.4067/s0716-97602003000100007. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fiber composition of the human corpus callosum: sex differences. Neuroreport. 1996;7:1761–1764. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- Aydinlioğlu A, Arslan K, Erdoğan AR, Rağbetli Ç, Keleş P, Diyarbakirli S. The relationship of callosal anatomy to paw preference in dogs. Eur J Morphol. 2000;38:128–133. doi: 10.1076/0924-3860(200004)38:2;1-f;ft128. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: a review of the evidence. Brain Lang. 1997;15:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. Neuroimage. 2001;13:1121–1130. doi: 10.1006/nimg.2001.0772. [DOI] [PubMed] [Google Scholar]
- Berrebi A, Fitch R, Ralphe D, Denenberg J, Friedrich V, Jr, Denenberg V. Corpus callosum: region-specific effects of sex, early experience, and age. Brain Res. 1988;438:216–224. doi: 10.1016/0006-8993(88)91340-6. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci Biobehav Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- Burke HL, Yeo RA. Systematic variations in callosal morphology: the effects of age, gender, hand preference, and anatomic asymmetry. Neuropsychology. 1994;8:563–571. [Google Scholar]
- Broadfield DC. Sex differences in the corpus callosum of Macaca fascicularis and Pan troglodytes. Columbia University; New York: 2001. p. 337. unpublished doctoral dissertation. [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca's area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher DL, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Lufkin RB, Zaidel E. Corpus callosum morphometry and dichotic listening performance: individual differences in functional interhemispheric inhibition? Neuropsychologia. 1993;31:547–557. doi: 10.1016/0028-3932(93)90051-z. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Zaidel E. Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav Brain Res. 1994;64:185–202. doi: 10.1016/0166-4328(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Fitch RH, Schrott LM, Cowell PE, Waters NS. Corpus callosum: interactive effects of infantile handling and testosterone in the rat. Behav Neurosci. 1991;105:562–566. doi: 10.1037//0735-7044.105.4.562. [DOI] [PubMed] [Google Scholar]
- Dorion AA, Chantome M, Hasboun D, Zouaoui A, Marsault C, Capron C, Duyme M. Hemispheric asymmetry and corpus callosum morphometry: a magnetic resonance imaging study. Neurosci Res. 2000;36:9–13. doi: 10.1016/s0168-0102(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Driesen NR, Raz N. The influence of sex, age, and handedness on corpus callosal morphology: a meta-analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- Dunham LD, Hopkins WD. Sex and handedness effects on corpus callosum morphology in chimpanzees (Pan troglodytes). Behav Neurosci. Forthcoming. doi: 10.1037/0735-7044.120.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger G. Hand preference, ability, and hemispheric specialization: in how far are these factors related to the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Kraemer GW, Shelton SE, Baker E, Kalin NH, Uno H. Gender differences in brain volume and size of corpus callosum and amygdale of rhesus monkey measured from MRI images. Brain Res. 2000;852:263–267. doi: 10.1016/s0006-8993(99)02093-4. [DOI] [PubMed] [Google Scholar]
- Foundas A, Leonard C, Heilman K. Morphological cerebral asymmetries and handedness: the pars triangularis and planum temporale. Arch Neurol. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Habib M, Gayraud D, Olivia A, Regis J, Salamon G, Khalil R. Effects of handedness and sex on the morphology of the corpus callosum: a study with brain magnetic resonance imaging. Brain Cogn. 1991;16:41–61. doi: 10.1016/0278-2626(91)90084-l. [DOI] [PubMed] [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Holloway RL, Anderson PJ, Defendini R, Harper C. Sexual dimorphism of the human corpus callosum from three independent samples: relative size of the corpus callosum. Am J Phys Anthropol. 1993;92:481–498. doi: 10.1002/ajpa.1330920407. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preference for bimanual feeding in 140 captive chimpanzees: rearing and ontogenetic determinants. Dev Psychol. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: cross-sectional analysis. J Comp Psychol. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. Int J Primatol. 1999;20:851–866. [Google Scholar]
- Hopkins WD. Hemispheric specialization in chimpanzees: evolution of hand and brain. In: Shackelford T, Keenan JP, Platek SM, editors. Evolutionary cognitive neuroscience. MIT Press; Boston: Forthcoming. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter AB, Pilcher DL. Grip morphology and hand use in chimpanzees (Pan troglodytes): evidence of left hemispheric specialization of motor skills. J Exp Psychol Gen. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling J, MacGregor L. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI). Neuroreport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Rilling JK. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: implication for the evolution of functional asymmetries. Behav Neurosci. 2000;114(4):739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees. Psychol Sci. 2005;16:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb Cortex. 1997a;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jancke L, Wunderlich G, Schlaug G, Steinmetz H. A case of callosal agenesis with strong anatomical and functional asymmetries. Neurosychologia. 1997b;35:1389–1394. doi: 10.1016/s0028-3932(97)00068-7. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol. 1990;291:520–537. doi: 10.1002/cne.902910404. [DOI] [PubMed] [Google Scholar]
- Larsen JP, Ødegaard H, Grude TH, Høien T. Magnetic resonance imaging—a method of studying the size and asymmetry of the planum temporale. Acta Neurol Scand. 1989;80:438–443. doi: 10.1111/j.1600-0404.1989.tb03906.x. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Manhães AC, Krahe TE, Caparelli-Dáquer E, Ribeiro-Carvalho A, Schmidt SL, Filgueiras CC. Neonatal transaction of the corpus callosum affects paw preference lateralization of adult Swiss mice. Neurosci Lett. 2003;348:69–72. doi: 10.1016/s0304-3940(03)00746-8. [DOI] [PubMed] [Google Scholar]
- Manhães AC, Medina AE, Schmidt SL, Filgueiras CC. Sex differences in the incidence of total callosal agenesis in BALB/cCF mice. Neurosci Lett. 2002;325:159–162. doi: 10.1016/s0304-3940(02)00292-6. [DOI] [PubMed] [Google Scholar]
- Manhães AC, Schmidt SL, Filgueiras CC. Callosal agenesis affects consistency of laterality in a paw preference task in BALB/cCF mice. Behav Brain Res. 2005;159:43–49. doi: 10.1016/j.bbr.2004.09.023. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in non human primates. Am J Phys Anthropol. 1997;104:201–232. [Google Scholar]
- Moffatt SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left-handers with evidence of left and right hemisphere speech lateralization. Brain. 1997;121:2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Olivares R, Michalland S, Aboitiz F. Cross-species and intra-species morphometric analysis of the corpus callosum. Brain Behav Evol. 2000;55:37–43. doi: 10.1159/000006640. [DOI] [PubMed] [Google Scholar]
- Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav Evol. 2001;57:98–105. doi: 10.1159/000047229. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of the commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheresonebrain: Functions of the corpus callosum. Alan R. Liss; New York: 1986. pp. 47–73. [Google Scholar]
- Rilling J, Insel T. Differential expansion of neural projection systems in primate brain evolution. Neuroreport. 1999;10:1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- Ringo J, Doty R, Demeter S, Simard P. Timing is of essence: a conjecture that hemispheric specialization arises from inter-hemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rosen GD. Cellular, morphometric, ontogenetic and connectional substrates of anatomical asymmetry. Neurosci Biobehav Rev. 1996;20:607–615. doi: 10.1016/0149-7634(95)00073-9. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sequeria SS, Woerner W, Walter C, Kreider F, Lueken U, Westerhausen R, Wittling RA, Wittling W. Handedness, dichotic-listening, and gender effects on planum temporale asymmetry—a volumetric investigation using structural magnetic resonance imaging. Neuropsychologia. 2006;44:622–636. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca's area homologue in African great apes: implications for language evolution. Anat Rec A Discov Mol Cell Evol Biol. 2003;271A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Smith RJ. Relative size versus controlling for size: interpretation of ratios in research on sexual dimorphism in the human corpus callosum. Curr Anthropol. 2005;46:249–273. [Google Scholar]
- Taglialatela J, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. Neuroreport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin G. Architecture of the precentral motor cortex and some adjacent areas. In: Bucy PC, editor. The precentral motor cortex. University of Illinois Press; Urbana, IL: 1949. pp. 7–82. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol Psychol. 1980;15:349–360. [Google Scholar]
- Witelson S. Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229:665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Nowakowski RS. Left out axons make men right: a hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia. 1991;29:327–333. doi: 10.1016/0028-3932(91)90046-b. [DOI] [PubMed] [Google Scholar]


