Abstract
It has been proposed that human right handedness is determined by genetic factors associated with the emergence of language, whereas non-human primate handedness is determined by random, non-genetic factors. These different mechanisms account for differences in the distribution of handedness between human and non-human primates. Here we report evidence that genetic factors play a role in the determination of handedness in chimpanzees. We further report that differential rearing has no influence on the expression of handedness in related individuals. Contrary to many theories of the origin of handedness, these results indicate that genetic factors have a significant influence on handedness in chimpanzees.
Keywords: handedness, laterality, chimpanzees
INTRODUCTION
Right-handedness is a universal trait in humans and some have suggested that it is a consequence of the left hemisphere's dominance for language and speech (Annett, 2002; Corballis, 2002). The universal expression of right-handedness in humans has led many to postulate a genetic basis for its expression (Annett, 2002; Corballis, 1997, 2006; Klar, 1999; McManus, 1985; McManus & Bryden, 1992), although at this point, no gene or set of genes has been explicitly linked to handedness (but see Francks et al., 2002). In contrast to human handedness, historically evidence of population-level handedness in non-human animals, particularly primates, has been weak (Warren, 1980). The lack of evidence for population-level handedness in mammals, notably primates, as well as the inability to selectively breed for directional paw preferences in mice, reinforced the view that laterality was unique to hominid evolution. Moreover, this contributed to the fundamental dichotomy between the mechanisms controlling the expression of hand preferences in human and non-human primates. Genetic factors determined human handedness, whereas non-genetic or random factors control the expression of non-human primate handedness.
Recently, evidence of population-level behavioral asymmetries in mammals, and specifically handedness, in nonhuman primates has challenged the assumptions of many genetic models of human handedness (Bradshaw & Rogers, 1993; Fagot & Vauclair, 1991; Hopkins, in press; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; Rogers & Andrew, 2002; Ward & Hopkins, 1993 but see McGrew & Marchant, 1997; Papademetriou, Sheu, & Michel, 2005). For example, in captive chimpanzees, population-level right handedness has been found for simple reaching, bimanual feeding, coordinated bimanual actions, manual gestures and throwing (see Hopkins, in press). In wild chimpanzees, evidence of population-level handedness has been reported for tool use (Biro et al., 2003; Boesch, 1991; Lonsdorf & Hopkins, 2005) and bimanual feeding (Corp & Byrne, 2004). The type of task used to assess handedness seems critical as findings in both captive and wild chimpanzees examining handedness for simple motor actions have failed to reveal evidence of population-level handedness (Finch, 1941; Fletcher & Weghorst, 2005; Marchant & McGrew, 1996; McGrew & Marchant, 2001; Papademetriou et al., 2005). The evidence of population-level handedness in great apes, for at least some measures, raises fundamental questions about the proposed mechanisms underlying the expression of handedness in human and non-human primates.
With respect to specific studies that have examined factors that influence handedness, there have been very few attempts to examine heritability of laterality in animals, and specifically, primates. The paucity of heritability studies in animals stems primarily from the historical lack of evidence of population-level asymmetries in non-human animals. Early selective breeding studies in mice failed to show increased incidences of paw preferences among genetically-selected lines of offspring (Collins, 1985) and these findings reinforced the view that the mechanisms determining human handedness were fundamentally different than those determining limb preferences in nonhuman animals. However, recent findings been shown that eye preferences for viewing predators in fish can be selectively bred (Bisazza, Facchin, & Vallortigara, 2000) and this breeding paradigm results in a suite of lateralized differences between genetic lines (see Vallortigara & Bisazza, 2002). In our own work with chimpanzees, we have reported that concordance rates in hand preferences among full- and maternal-half siblings living together or apart are significantly above chance (Hopkins, 1999b; Hopkins, Dahl, & Pilcher, 2001). In recent studies in wild chimpanzees, it was also shown that maternal and offspring hand preferences were significantly associated as were concordance rates in hand preference among maternal half-siblings (Lonsdorf & Hopkins, 2005; Matsuzawa et al., 2001). Lastly, Annett (in press) applied her statistical analyses as they pertain to the right shift genetic theory of handedness to a sample of data from chimpanzees and suggested that chimpanzees were shifted rightward, although the magnitude of expression was significantly less than the results found in humans. This suggests a possible genetic basis for handedness in chimpanzees but the representation of the potential genes differs from humans.
In both the human and non-human primate handedness literature, there have been little to no attempts to empirically assess the interaction between genetic and non-genetic factors on the development of handedness. In particular, the potential role of prenatal and post-natal factors as modulators of the phenotypic expression of handedness has not been examined to date in human subjects. In light of the fact that heritability estimates of human handedness do not explain a large proportion of the variance, it might suggest that considering non-genetic factors in relation to familial handedness might improve the degree of variability accounted for by genetic variables.
One purpose of this study was to evaluate heritability in the handedness of chimpanzees in relation to the role of prenatal and perinatal factors. Specifically, in previous research, we have found that there is a higher proportion of left-handedness among 1st and latter-born (parities >6) chimpanzees (Hopkins & Dahl, 2000). Moreover, we have previously found that concordance rates among maternal half-siblings are significantly higher when adjusted for the parity of the offspring. That is, concordance rates are significantly higher among non-first born siblings compared to siblings in which one of the offspring is a 1st born (Hopkins et al., 2001). This finding was based on a sample of chimpanzees housed at the Yerkes National Primate Research Center and since that time, we have collected data on two additional colonies of apes. Thus, the findings presented here represent data from a larger cohort of chimpanzees and allows for an elaborate investigation of the influence of perinatal and genetic factors on handedness in chimpanzees.
In addition, in the current study, we evaluated the role of genetic compared to social learning explanations for the development of handedness in a sample of captive chimpanzees. Specifically, in wild chimpanzees, Lonsdorf and Hopkins (2005) found evidence that hand preferences for tool use run in families. Significant concordance rates were found in hand preferences between mothers and their offspring as well as between siblings. Notwithstanding, the evidence of heritability in hand preferences in wild chimpanzees for tool use cannot isolate the potential role of genetics relative to social learning because the two variables are confounded. The issue of social learning is particularly relevant because it has been shown that social learning plays an important role in the development of termite fishing in wild chimpanzees (Lonsdorf, 2005).
One advantage of studying captive chimpanzees is that the influence of genetic and non-genetic factors (such as rearing) can be isolated to assess their independent or interactive effect on behavioral development. For example, with respect to handedness, in previous studies, Hopkins et al. (2001) reported that genetically related individuals raised together or apart did not statistically differ in concordance rates in hand preference. This result, however, was contingent upon consideration of risk factors associated with pregnancy and parturition in the chimpanzees. Rather than focus solely on perinatal or maternal factors, in this study, we were specifically interested in examining the handedness of related and unrelated chimpanzees living together or apart. Our hypothesis was that if genetic factors play a role in determining the handedness of chimpanzees, then genetically related individuals, regardless of whether they are living together or apart, should be more similar in their handedness than should unrelated individuals.
METHODS
Subjects
The subjects initially tested were 467 captive chimpanzees housed at three facilities in North America including the Yerkes National Primate Research Center (YERKES), the University of Texas M. D. Anderson Cancer Center (UTMDACC) and the Alamogodo Primate Facility (APF). The number of animals included in the analyses described below varied according to the social housing condition and relatedness of individuals living within a group.
Procedure
Handedness data were collected on a measure requiring coordinated bimanual actions, referred to as the TUBE task (see Hopkins, 1995) (see Fig. 1). Briefly, peanut butter is smeared on the inside edges of a hollow, poly-vinyl-chloride (PVC) pipe, approximately 20 cm in length. The PVC pipe was then handed to the subjects in their home cage and the finger/hand used in removing the peanut butter was recorded by the observer as either left or right. The hand active in removing the peanut butter was defined as the dominant hand. Observations continued until the subjects had eaten all the food, had dropped the tube, or had handed the tube back to the experimenter. A minimum of 20 responses were recorded from each subject. Although other measures of hand preference have been collected in these chimpanzees (Hopkins, Wesley, Izard, Hook, & Schapiro, 2004), the TUBE measure was used to classify subjects as left- or right-handed because: (a) it induces individual hand preferences in more than 85% of the chimpanzees; and (b) it is a highly reliable measure with test-retest measures exceeding 0.70 over a 2-year period (Hopkins, 2006).
FIGURE 1.

Picture of a chimpanzee engaged in the TUBE task which measures coordinated bimanual actions. Note that the chimpanzee is holding the tube with one hand (left) and extracting food with the opposite hand (right).
Data Analysis
Frequency of left and right hand use were recorded for each subject and a handedness index (HI) was derived following the formula [HI = (#R–#L)/(#R+#L)] with values ranging from −1.0 to 1.0. Subjects with a negative score or a score of zero were classified as non-right-handed and all others were classified as right-handed. This is a very liberal criterion for hand preference classification but it was adopted to simplify the analysis and to eliminate the issue of “ambidextrous” individuals. Moreover, reducing the number of handedness classifications increased our statistical power. Based on these categorical data, chimpanzees with the same classification of hand preference were considered concordant while all others were considered disconcordant. Subjects were also categorized into three groups based on their birth order. Group 1 consisted of first born animals, Group 2 consisted of chimpanzees with parities from two to five, and Group 3 consisted of chimpanzees with parities of six or higher. Rearing served as an additional variable in some analysis with mother-reared subjects being raised by their biological mothers and nursery-reared subjects being raised by humans in a nursery setting from at least the 30th day of life.
RESULTS
Subject Variable Effects on Handedness
For this analysis, the HI scores were subjected to a four-factor between group analysis of variance. Colony (YERKES, UTMDACC, APF), birth order group (groups 1−3), rearing (mother-reared, nursery-reared) and sex (male, female) were the independent variables. A significant main effect for birth order was found F(3,308)=3.26, p<.04, a finding consistent with our previous studies there were restricted to the YERKES chimpanzees. Post-hoc analysis indicated that the mean HI scores for group 2 (M=.20, s.e.=.04) were significantly higher than the HI scores for group 1 (M=−.039, s.e.=.11) and group 3(M=.045, s.e.=.075). No other significant main effects or interactions were found.
Consistent with our previous work, because birth order groups 1 and 3 did not significantly differ with respect to their HI scores, we subsequently collapsed this group of subjects and referred to them as the perinatal risk group. Subjects in birth order group 2 remained the same but were renamed the perinatal non-risk group. This distinction in birth groups was used in the subsequent analyses examining the interaction between maternal and paternal handedness and perinatal factors on offspring handedness.
Maternal and Paternal Effects on Handedness
Based on the sign of their HI value, offspring, their dams and their sires were classified as right- or non-right-handed. We compared the distribution of handedness between offspring and maternal and paternal handedness using chi-square tests of independence. Maternal handedness was known for 198 offspring and paternal handedness was known for 110 offspring. No significant associations were found between offspring and either maternal or paternal handedness. Rather than evaluate the independent association between offspring and maternal and paternal handedness, we also classified parental handedness as either both right-handed (n=34), both non-right-handed (n=21) or mixed handedness (n=40) and compared the distribution of offspring handedness. No association was found between offspring and parental handedness. The overall distribution of non-right and right-handed offspring born to the different cohorts is shown in Table 1.
Table 1.
Distribution of Offspring Handedness In Relation to Parental Handedness
| Offspring Handedness | ||
|---|---|---|
| Non-right-handed | Right-handed | |
| Parental handedness | ||
| Both non-right-handed | 7 | 14 |
| Mixed | 13 | 27 |
| Both right-handed | 11 | 23 |
| Maternal | ||
| Non-right-handed | 21 | 41 |
| Right-handed | 41 | 95 |
| Paternal | ||
| Non-right-handed | 15 | 38 |
| Right-handed | 18 | 39 |
Rearing and Parental Handedness
For this analysis, each mother-offspring and father-offspring dyad was classified as being concordant or disconcordant based on the sign of their HI values. We then compared the distribution of concordance in hand preference as a function of whether the offspring was raised by their conspecific mother or human-reared. No significant associations were found between rearing and maternal or paternal handedness. For mother-offspring dyads, 63% of mother-reared individuals were concordant compared to 58% for human-reared. For the father-offspring dyads, 52% of mother-reared offspring were concordant whereas 47% of human-reared offspring had the same hand preference.
Perinatal Risk and Parental Handedness
For this analysis, as with the previous analysis, each mother-offspring and father-offspring dyad was classified as being concordant or disconcordant based on the sign of their HI value. We then compared the distribution of concordance in hand preference as a function of perinatal risk group as indicated by the subject's parity (non-risk, risk). These data are shown in Table 2. A significant association was found between perinatal risk and maternal handedness X2(1, n=197)=4.12, p<.05 but not paternal handedness X2(1, n=110)=0.89, n.s. For mother-offspring dyads, a significantly higher proportion of offspring born in the non-risk group were concordant for handedness compared to offspring born to the risk group.
Table 2.
Distribution of Concordance Rates in Handedness Between Offspring and Parents as a Function of Perinatal Risk
| Disconcordant | Concordant | R:L odds | |
|---|---|---|---|
| Maternal | |||
| Non-risk | 30 | 86 | 2.87 |
| Risk | 32 | 49 | 1.53 |
| Paternal | |||
| Non-risk | 16 | 38 | 2.38 |
| Risk | 21 | 34 | 1.61 |
Because our main interest in this study was in the potential interaction between genetic and perinatal risk factors, we performed some additional analysis to provide at least a preliminary examination of these possible associations using more powerful statistics than chi-square. For this analysis, we classified the handedness of both parents as either (a) both right-handed, (b) both non-right-handed, and (c) mixed dominant (one right-handed, none non-right-handed). We then compared the HI scores of the offspring born to each of these cohorts as a function of whether they were classified into the non-risk or risk parity. The mean HI scores for offspring born to each parental cohort as a function of perinatal risk group is shown in Figure 2. For chimpanzees born to two right-handed t(32)=2.35, p<.03 or mixed-handed t(38)=2.29, p<.04 parents, non-risk offspring had significantly higher HI scores than risk offspring. For offspring born to two non-right-handed parents, there was no significant difference in their HI scores.
FIGURE 2.
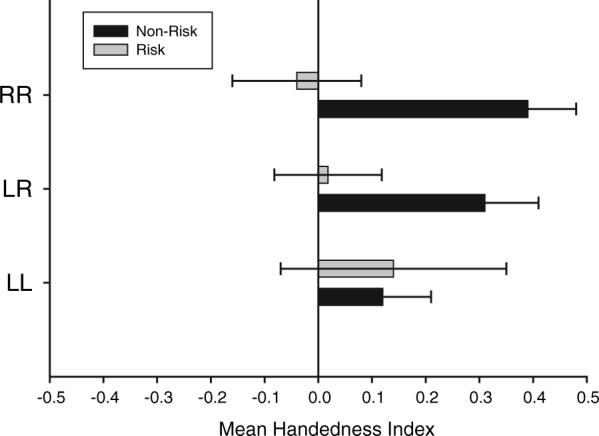
Mean HI (±s.e.) scores for risk and non-risk offspring born to two right-handed (RR), two left-handed (LL) or mixed handed (RL) parents. Risk offspring had a parity of 1 or 6 or higher. Non-risk offspring had parities between 2 and 5.
Additional Heritability Analyses
To more fully exploit the existing pedigrees at each facility as well as simulate some of the social circumstances of wild chimpanzees, we performed some additional analyses to assess the potential role of social learning and genetics on handedness in the chimpanzees. These analyses were restricted to social groups of animals with six or more members in the three colonies.
In the initial analyses, within different social groups based on the known pedigrees, we identified related and unrelated individuals living in the same groups and compared hand preferences for the TUBE task. Specifically, in socially housed chimpanzees of six or more individuals, the HI values of each focal chimpanzee in the group was compared to the average HI for all chimpanzees (a) related to them and living in the same group, (b) all chimpanzees unrelated to them and living in the same group, and (c) chimpanzees related to them but not living in the same group. The mean HI for each cohort is shown in Figure 3. The mean HI scores of individual chimpanzees did not significantly differ from related individuals living in the same group t(110)=0.61, n.s., related individual living outside the group t(88)=1.167, n.s. but did differ significantly from unrelated individuals living in the same group t(111)=2.05, p<.04. In addition, individuals related to the focal subject living in the same group had significantly higher HI scores than unrelated individuals living in the same group t(111)=2.48, p<.02 but did not differ from related individuals living outside the group t(97)=0.41, n.s. Thus, chimpanzee HI scores for the TUBE task were more similar to related-compared to unrelated individuals living in the same or a different social group.
FIGURE 3.
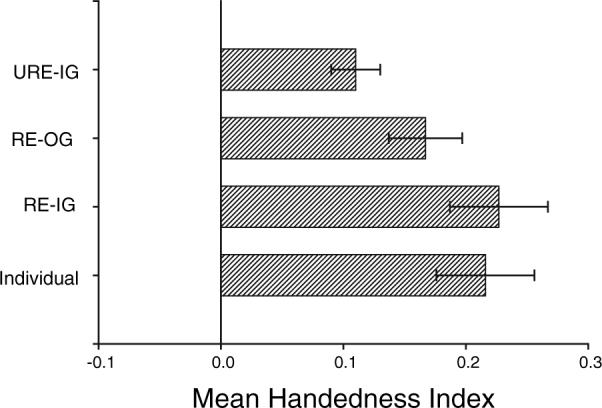
Mean HI (±s.e.) scores for unrelated chimpanzees living in the same social group (URE-IG), related individuals living in a different group (URE-OG), related individuals living in the same group (RE-IG) and all unrelated and related individuals in the sample (Individual).
To further examine heritability in handedness, in the next analysis we identified adult females living in each of these groups and classified their handedness as right (HI values >0, n=22) or left (HI≤0, n=12). We then calculated the mean HI for all individuals related to each female living in the same group (FAM-HI) and the mean HI for unrelated individuals living in the same group (UNFAM-HI). The FAM-HI and UNFAM-HI served as repeated measures in a mixed model ANOVA with maternal handedness serving as the between group factor. A significant two-way interaction was found between maternal handedness and the related HI variable F(1,32)=9.32, p<.001. Shown in Figure 4 are the mean FAM-HI and UNFAM-HI values for left- and right-handed females. Offspring of left-handed females had lower HI scores than unrelated individuals living in the same group. In contrast, offspring born to right-handed females had higher HI scores than unrelated individual living in the same group.
FIGURE 4.
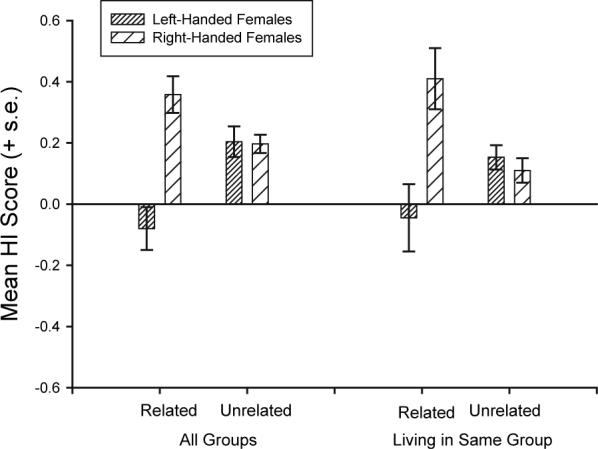
Mean HI scores (±s.e.) for offspring born to right- or left-handed females compared to unrelated individuals living in the same group. For one analysis, all individuals were included whereas in the second analysis, only social groups in which both a left- and right-handed females were living were included.
In the previous analysis, within a social group, it could be argued that there may not have been both left- and right-handed individuals living in the same group and therefore the previous results may simply reflect founder effects within a social group. To address this issue, we performed the exact same analysis as described above, but restricted it to groups in which at least one left- and one right-handed female resided. This reduced the sample size to seven left-handed females and nine right-handed females, yet, despite the reduction in statistical power, a significant interaction was found between maternal handedness and the relatedness of group members F(1,14)=8.68, p<.01. Shown in Figure 4 are the mean FAM-HI and UNFAM-HI values for left- and right-handed females. The handedness of offspring largely conforms to the handedness of their biological mother, despite living among individuals that display other hand preferences.
DISCUSSION
The study revealed several significant findings. First, birth order has a significant effect on handedness in captive chimpanzees. Second, hand preferences run in families of chimpanzees, and specifically, there is a relatively strong maternal effect on handedness. Third, to some extant, heritability in handedness is modulated by birth order.
With respect to birth order, the evidence of increased left-handedness among 1st and latter-born chimpanzees revealed in this study (which included more than 300 chimpanzees from two additional facilities) is consistent with previous results based on the YERKES chimpanzees alone (see Hopkins & Dahl, 2000). This suggests that the earlier observations are not restricted to the YERKES colony and generalize to at least two other colonies of apes. Birth order per se is probably not the likely factor but merely reflects potentially other kinds of pregnancy risk variables, such as maternal age, gestational age of the offspring, birth stress, or birth trauma, to name a few, that might contribute to this finding (see Bailey & McKeever, 2004). Which of these myriad of pregnancy or periparturitional variables is critical for influencing the development of laterality remain unknown but warrants further investigation. In the human handedness literature, discrepancies in findings on the effect of birth order on hand use center around sampling biases and whether relatively homogenous or heterogeneous samples are obtained with respect to socio-economic or other socio-cultural variables. It is difficult to interpret the results in the chimpanzees from this perspective; thus, we believe the results in the chimpanzees reflect the influence of pre- and perinatal factors on the development of handedness of chimpanzees.
It should also be noted that there are some differences in the characterization of birth order between the studies in chimpanzees compared to humans. In this study, birth order was based on the gravis of the female which included all incidences of stillbirth or miscarriage in the determination of an individuals' birth order. Thus, literal parity was not used to characterize birth order but rather birth order was based on the inclusion of all pregnancies that resulted in a live or still birth or were prematurely terminated. As far as we know, studies in human subjects do not include stillbirth or miscarriages as part of the determination of birth order but rather rely on the self-reported parity of the offspring based on live births. A more fully characterized reproductive history of the mothers of subjects in handedness studies might produce more reliable results in human populations. On the contrary comparative side, with increasingly available longitudinal data in feral apes, including reproductive histories, it would be interesting to assess whether parity influences the handedness of wild chimpanzees.
Several findings in this study support the interpretation that there is a significant maternal effect on the development of handedness in chimpanzees. First, HI scores among related individuals living in the same group are more similar to their biological mothers than unrelated individuals living in the same group. Second, a significant association was found between concordance rates in hand preference between offspring and their mother, when birth order was accounted for in the analysis. Paternal handedness has no significant influence on offspring hand preference, independently or in relation to birth order. Why the maternal but not paternal effect is unclear but several hypotheses are possible including social learning or early positional asymmetries. Regarding social learning, it might be that offspring model the hand use seen in their mothers. Although social learning cannot be ruled out, it is difficult for a social learning explanation to account for the lack of statistical difference in concordance rates between offspring and mothers reared together or apart. Moreover, one can hardly argue that social learning would be strongly needed to solve the TUBE task, which is relatively simple, compared to more complex behaviors such as tool use. An alternative explanation for the maternal effect on handedness is the hypothesis that early asymmetries in development imposed by the cradling bias of the mother influences the development of handedness of the offspring (see Hopkins, 2004 for details). Great apes show laterality in cradling biases (see Damerose & Vauclair, 2002; Hopkins, 2004; Manning, Heaton, & Chamberlain, 1994 for reviews) and it has been shown that the early laterality in cradling inversely predicts the subsequent handedness of offspring tested 4 years later (Hopkins, in press). Thus, offspring born to females that cradle their offspring on the right develop left-hand preferences while offspring born to females that cradle their offspring on the left develop right handedness. Assuming that the females cradle with their non-dominant hand in order to keep their preferred hand free for other activities, such as locomotion or manipulation, the offspring subsequently develop the same hand preference of their mothers, not through social learning, but because of the early asymmetric stimulation imposed by the mother. Lastly, the evidence of heritability in hand preferences in chimpanzees does not rule out the possibility that they are under some genetic control but in the absence of any specific candidate genes this remains as speculative as any existing human genetic model. With the recent publication of the chimpanzee genome (The Chimpanzee Sequencing and analysis Consortium, 2005), the possibility of identifying potential genes through molecular genetic techniques or quantitative trait linkage has improved dramatically and this line of inquiry should be pursued.
One significant limitation of this study, and others, is the limited amount of data on paternal handedness. Data from sires are more difficult to obtain because there are simply fewer living individuals in the various social groups and this limits the kinds of heritability analyses that can be performed, particularly when considering the combined maternal and paternal effects of handedness. One of the main reasons for the lack of paternal data is that male chimpanzees have a much shorter lifespan than females (14 years by some estimates, Dyke, Williams-Blangero, Mameleka, & Goodwin, 1996). Moreover, there is a reasonably long-standing moratorium on breeding of captive chimpanzees in NIH-supported research facilities and therefore younger reproductively fertile males for which handedness data are known have not produced offspring that can subsequently be tested for hand use. Collectively, this suggests that there is an increasing need to test additional colonies of apes in order to increase the amount of paternal handedness data to more fully examine heritability of handedness in chimpanzees. Similarly, in wild chimpanzees, most heritability analyses have been restricted to offspring-maternal or sibling analyses due to limited knowledge of paternity in many individuals. With increasingly sophisticated techniques available for extraction of DNA from biological materials in wild chimpanzees, this will provide a means of determining paternity in different individuals and expand the scope of heritability analyses in feral apes.
In conclusion, the results of this study further reinforce the view that handedness is heritable in chimpanzees and particularly there appears to be good evidence for a maternal effect on hand preference. To some extent, perinatal factors modulate the expression of heritability of handedness in chimpanzees. Similar studies are not readily available in human subjects but within-family variation in handedness in relation to perinatal factors would be an important contribution to extant genetic models of handedness in humans. These data would foster comparison of heritability estimates between chimpanzees and humans and speak more directly to the role of genetic and non-genetic mechanisms influencing the development of behavioral and brain asymmetries in primates.
NOTE
Contract grant sponsor: NIHRR-00165, U42-RR-15090, NS-42867, NS-36605 HD-38051
This work was supported in part by NIH grants RR-00165, U42-RR-15090, NS-42867, NS-36605 and HD-38051.
REFERENCES
- Annett M. The distribution of handedness in chimpanzees: Estimating right shift in Hopkins' sample. Laterality. 2006;11:101–109. doi: 10.1080/13576500500376500. [DOI] [PubMed] [Google Scholar]
- Annett M. Handedness and brain asymmetry: The right shift theory. Psychology Press; Hove: 2002. [Google Scholar]
- Bailey LM, McKeever WF. A large-scale study of handedness and pregnancy/birth risk events: Implications for genetic theories of handedness. Laterality. 2004;9:175–188. doi: 10.1080/13576500342000013. [DOI] [PubMed] [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Animal Cognition. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Facchin L, Vallortigara G. Heritability of lateralization in fish: Concordance of right-left asymmetry between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bradshaw B, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. Academic Press; San Diego: 1993. [Google Scholar]
- Collins RL. On the inheritance of direction and degree of asymmetry. In: Glick S, editor. Cerebral lateralization in non-human species. Academic Press; Orlando: 1985. pp. 150–164. [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From hand to mouth: The origins of language. Princeton University Press; Princeton: 2002. [Google Scholar]
- Corballis MC. Cerebral asymmetry: A question of balance. Cortex. 2006;1:116–117. doi: 10.1016/s0010-9452(08)70335-6. [DOI] [PubMed] [Google Scholar]
- Corp N, Byrne RW. Sex difference in chimpanzee handedness. American Journal of Physical Anthropology. 2004;123:62–68. doi: 10.1002/ajpa.10218. [DOI] [PubMed] [Google Scholar]
- Damerose E, Vauclair J. Posture and laterality in human and non-human primates: Asymmetries in maternal handling and infant's early motor asymmetries. In: Rogers L, Andrews R, editors. Comparative vertebrate lateralization. Oxford University Press; Oxford: 2002. pp. 306–362. [Google Scholar]
- Dyke B, Williams-Blangero S, Mameleka PM, Goodwin WJ. Future costs of chimpanzees in US research institutions. ILAR. 1996;37:193–198. doi: 10.1093/ilar.37.4.193. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in primates. A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Finch G. Chimpanzee handedness. Science. 1941;94:117–118. doi: 10.1126/science.94.2431.117. [DOI] [PubMed] [Google Scholar]
- Fletcher AW, Weghorst JA. Laterality of hand function in naturalistically housed chimpanzees (Pan troglodytes). Laterality. 2005;10:219–242. doi: 10.1080/13576500442000049. [DOI] [PubMed] [Google Scholar]
- Francks C, Fisher SE, MacPhie L, Richardson AJ, Marlow AJ, Stein JF, Monaco AP. A genomewide linkage screen for relative hand skill in sibling pairs. American Journal of Human Genetics. 2002;70:800–805. doi: 10.1086/339249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in non-human primates. International Journal of Primatology. 1999a;20:851–866. [Google Scholar]
- Hopkins WD. Heritability of hand preference in chimpanzees: Evidence from a partial interspecies crossfostering study. Journal of Comparative Psychology. 1999b;113:307–313. doi: 10.1037/0735-7036.113.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Laterality in maternal cradling and infant positional biases: Implications for the evolution and development of hand preferences in nonhuman primates. International Journal of Primatology. 2004;25:1243–1265. doi: 10.1023/B:IJOP.0000043961.89133.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee right-handedness: Internal and external validity in the assessment of hand use. Cortex. 2006;42:90–93. doi: 10.1016/s0010-9452(08)70326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF. Birth order and hand preference in chimpanzees (Pan troglodytes): Implications for pathological models of human handedness. Journal of Comparative Psychology. 2000;114:302–306. doi: 10.1037/0735-7036.114.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): Evidence in support of the right shift theory and developmental instability. Psychological Science. 2001;12:299–303. doi: 10.1111/1467-9280.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees are predominantly right-handed: Replication in three colonies of apes. Behavioral Neuroscience. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ. Genetic models of handedness, brain lateralization, schizophrenia, and manic-depression. Schizophrenia Research. 1999;39:207–218. doi: 10.1016/s0920-9964(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV. Sex differences in the development of termite-fishing skills in wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Animal Behaviour. 2005;70:673–683. [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population-level handedness for tool use.; Proceedings of the National Academy of Sciences; 2005. pp. 12634–12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Manning JT, Heaton R, Chamberlain AT. Left-side cradling: Similarities and differences between apes and humans. Journal of Human Evolution. 1994;26:77–83. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- Matsuzawa T, Biro D, Humle T, Inoue-Nakamura N, Tonooka R, Yamakoshi G. Emergence of culture in wild chimpanzees: Education by master-apprenticeship. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Springer; Tokyo: 2001. pp. 557–574. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- McManus C. Handedness, language dominance, and aphasia. A genetic model. Psychological Medicine. 1985;18:347–355. [PubMed] [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of neuropsychology. Vol. 6. Elsevier; Amsterdam: 1992. pp. 115–144. (Developmental neuropsychology, Part 1.). [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences for reaching and other hand-use measures. Journal of Comparative Psychology. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew RJ. Comparative vertebrate lateralization. Cambridge: Cambridge University Press. The Chimpanzee Sequencing and analysis Consortium. (2005). Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2002;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Bisazza A. How ancient in brain lateralization? In: Rogers LJ, Andrew RJ, editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge: 2002. pp. 9–69. [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]


