Abstract
Studies of great apes have revealed that they use manual gestures and other signals to communicate about distal objects. There is also evidence that chimpanzees modify the types of communicative signals they use depending on the attentional state of a human communicative partner. The majority of previous studies have involved chimpanzees requesting food items from a human experimenter. Here, these same communicative behaviors are reported in chimpanzees requesting a tool from a human observer. In this study, captive chimpanzees were found to gesture, vocalize, and display more often when the experimenter had a tool than when she did not. It was also found that chimpanzees responded differentially based on the attentional state of a human experimenter, and when given the wrong tool persisted in their communicative efforts. Implications for the referential and intentional nature of chimpanzee communicative signaling are discussed.
Keywords: Tool use, Cognition, Communication, Chimpanzee, Manual gesture
Studies have revealed that like humans, wild and captive great apes use manual gestures to communicate about distal objects (e.g. Bard 1992; Call and Tomasello 1994; Goodall 1986; Leavens and Hopkins 1999; Pika et al. 2003; Schaller 1963; Tanner and Byrne 1996, 1999). In captive apes, there is now substantial evidence to suggest that some manual gestures are intentional communicative signals (Leavens et al. 1996; Leavens et al. 2004). The first set of data supporting this claim is based on studies showing that chimpanzee gesturing is very often accompanied by gaze alternation between the human experimenter and the desired object (Leavens and Hopkins 1998; Leavens et al. 2004; Krause and Fouts 1997). This is a significant finding considering that gaze alternation is a commonly used criterion to define intentional communication in preverbal human children (e.g. Harding and Golinkoff 1979). The second set of supporting data is based on the fact that chimpanzees recognize the need to acquire a human experimenter's attention before gesturing to the desired object (Hostetter et al. 2001; Krause and Fouts 1997). Povinelli and Eddy (1996) found that even young chimpanzees preferred to request food from an experimenter who was making eye contact with them rather than a human who was looking away. Krause and Fouts (1997) observed that their two chimpanzee subjects used various sounds including foot stomping, cage banging, vocalizations, and clapping to attract the attention of the human experimenter before pointing to a desired but out of reach object.
One limitation in the bulk of previous studies on gesturing in captive apes is that the distal object has almost always been food (e.g. Bard 1992; Leavens et al. 1996; Krause and Fouts 1997). It is possible that the emotional arousal that inevitably follows the visual presence of food may play a role in the resultant communicative behaviors. In this scenario, the subject makes noises such as cage banging or vocalizing as a result of the frustration and excitement caused by the visual presence of unattainable food, not to acquire the joint attention of the human experimenter per se. Similarly, it has been argued that some gestures produced by captive apes are frustrated reaching attempts toward the otherwise unattainable food (see Leavens and Hopkins 1999), although the absence of their production when no human is present does not support this argument (the so-called audience effect). In short, the question of whether or not out of reach food is the only context in which these communicative behaviors occur remains largely unanswered.
The main objective of the current study was to determine if chimpanzees will engage in communicative signaling in the presence of a desirable non-food object, specifically a tool needed to obtain food. Call and Tomasello (1994) addressed this question in a study of two orangutans (Pongo pygmaeus) who were required to gesture to a hidden tool in the presence of a human experimenter. Only one of the two subjects, Chantek an enculturated orangutan, was successful in this paradigm, suggesting that specific language-training and extended contact with humans may be a necessary condition for the productionof communicative signals to non-food objects. The current study was designed to address this issue in a much larger sample of chimpanzees using a simpler paradigm. The current design required subjects to obtain a tool from the human experimenter in order to access food from a honey dipping device placed in their enclosure. The human holding the tools was located in a different location from the cage containing the tool device. Note that in this situation, the food itself was neither visible to the chimpanzee nor obtainable by the human during the chimpanzee–human interactions. If the chimpanzees exhibit gestural, vocal, and other attention-getting behaviors in an attempt to retrieve a tool from a human experimenter, then this would suggest that these behaviors are truly communicative in function and not just a reaction to out of reach, unattainable food.
A second objective of this study stemmed from research showing that chimpanzees are sensitive to the attentional state of a human communicative partner (Hostetter et al. 2001) when producing different communicative signals. Specifically, chimpanzees use more visual modes of communication such as gestures and lip pouts when an experimenter is facing them and has food than when the experimenter has her back turned towards the subject but is still holding food. In addition, the chimpanzees produced their first vocalization and spit faster in the “away condition” than in the “toward condition,” while the opposite was true for the behaviors gesture and lip pout. Our aim was to see if we could replicate this effect using a tool instead of food as the object of interest.
The final goal of the current study was to examine the degree to which chimpanzees' communication about tools is intentional and goal-directed. Leavens et al. (2005) found that when chimpanzees were given chow or a half banana instead of the whole banana they desired, the subjects persisted and elaborated in their communicative efforts to obtain their goal. In the current study, a similar scenario is presented using an effective and ineffective tool. If the chimpanzees' communication is goal-directed, then they should continue to communicate with the experimenter after being given an ineffective tool compared to an effective tool.
Experiment 1
Method
Subjects
Subjects were 26 chimpanzees (Pan troglodytes) housed at Yerkes National Primate Research Center (hereafter referred to as Yerkes), Atlanta, Georgia and 91 chimpanzees housed at The University of Texas M.D. Anderson Cancer Center (hereafter referred to as Bastrop), Bastrop, Texas. Of the total 117 subjects, 62 were females and 55 were males. Subjects ranged in age from 6 to 46 years (mean±SD=22.00±10.86). Forty-two of the subjects were mother-reared, 37 were nursery-reared, and 38 were wild-caught. Mother-reared and nursery-reared subjects were both born into captivity and were either raised by their biological mothers for the initial 30 days of life or sent to the nursery before 31 days, respectively. Wild caught animals were obtained from their natural habitat in Africa.
The housing conditions varied across subjects. All of the Yerkes animals were pair or socially housed in indoor/outdoor runs. Housing conditions varied for Bastrop subjects. Approximately 20 of these subjects were pair or group housed in indoor/outdoor runs similar to Yerkes, while the rest were socially housed in large outdoor primadomes or corrals with connected indoor cages. The indoor/outdoor runs at both facilities had concrete floors, cinderblock walls, perches, and enrichment toys. The outdoor primadomes and corrals had natural, grassy flooring with natural objects such as leaves and sticks available along with other enrichment structures and toys.
All subjects were experienced tool users and were familiar with the honey dipping device used in this study (see Fig. 1). At Yerkes, the honey dipping device consisted of three PVC pipes (15 cm long, 4 cm in diameter) each glued at a 45° angle into three holes (4 cm in diameter) placed horizontally, 15 cm apart on a rectangular plastic board (50 cm long × 20 cm wide). The device used at Bastrop followed the same design, but was made with a metal box instead of a plastic board and the PVC pipes were slightly longer. One end of each tube (the one glued to the rectangular plastic or metal board) was open to allow access to the honey down at the other end, which in turn was closed by a removable PVC cap.
Fig. 1.

Adult male chimpanzee using a reed to retrieve honey from a Yerkes tool use device
At the Yerkes facility, the honey dipping device was attached with stainless steel screws onto the cage wall of the subjects' home cage prior to each test and removed once the subjects had exhausted the honey. At the Bastrop facility, the honey dipping devices were permanently attached to the enclosure and would simply be baited at the beginning of each test session. Subjects could access the honey by dipping a tool through the mesh into the PVC pipe. The tools used at the Yerkes facility were reed sticks measuring approximately 18 cm long and 0.05 cm in diameter. The tools used at Bastrop were fresh cut bamboo reeds which were approximately 18 cm long and 1 cm in diameter.
Procedure
The basic protocol was for an experimenter to bait the honey dipping device(s) in one part of the cage with the animals watching and then leave the area for a minimum of 60 s. Subjects were not separated from their social groups for testing. For the tool condition, the experimenter returned in full view of the subjects and sat approximately 1 m from the mesh at a point away from the area of the honey dipping device with a handful of tools (approximately 20 reed sticks) visible to the subjects. The experimenter then observed and recorded behaviors (gesture, vocalization, spit, clap, cage bang, rump present, display, barter, and throw) for a period of 5 min (see Hostetter et al. 2001 for a complete description of the behavioral ethogram used here). Note that gestures included whole-hand pointing, food begs, and single-digit pointing. Along with specific behaviors, the experimenter also recorded the time elapsed from the onset of the observation period until the first behavioral response occurred for each subject. At the end of the observation period, all subjects were given a tool and allowed to use the honey dipping device. The no-tool condition proceeded in the same way except that the experimenter did not have any tools present during the observation period. The experimenter just simply sat in front of the cage without any tools in her hand. At the end of the observation period the experimenter retrieved the tools and gave one to each subject. In both conditions, the experimenter had a clipboard and stop watch with them to reduce the possibility that having any object, not necessarily a tool, was enough to elicit communicative behaviors. It should be noted that each subject received only one trial in each condition, so the results represent first trial data. However, although this task was unique for all the subjects, many of the Yerkes chimpanzees had participated in previous studies on gestural communication in similar types of paradigms (see Hostetter et al. 2001; Leavens et al. 2004). The order in which the tool and no-tool conditions were administered was counterbalanced across subjects.
The exact procedure varied slightly depending on the type of housing. The goal in each housing condition was to require the animal to make a deliberate and overt attempt to communicate with the experimenter by requiring them to move to an area of their enclosure away from the honey dipping device. This attempt was made to reduce the likelihood that the subject was exhibiting an emotional response to the presence of unattainable food. For pair-housed subjects who had access to only one outdoor and one indoor cage, the honey dipping device was placed on the outside cage and the experimenter was located in front of the indoor cage. Thus, the animals had to move through an open door connecting the indoor and outdoor enclosures to communicate with the experimenter.
For group-housed individuals living in an indoor/outdoor run with access to more than one cage, the honey dipping device was placed on one outside cage and the experimenter was located in front of the adjacent outdoor cage. The door between the two outdoor cages was closed prior to testing so that subjects had to move through an open door to the inside, through an open door to the adjoining indoor cage, and through another open door to the opposite outdoor cage in order to reach the experimenter's location.
For subjects housed in outdoor primadomes and corrals, the honey dipping devices were placed and baited on one side of the enclosure with the experimenter being located on the opposite side of the enclosure for testing. Thus subjects had to move away from the honey dipping device and across the enclosure to communicate with the experimenter and request the tool.
Data analysis
For the purpose of statistical analysis, cage bang, spit, clap, and throw were combined together and referred to as attention-getting behaviors. A difference score was calculated by subtracting the total number of responses in the no-tool condition from the total number of responses in the tool condition such that positive values represent more communication in the tool than the no-tool condition. A univariate analysis of variance (ANOVA) was then used to evaluate the effects of sex and rearing on the subjects' communicative behaviors. A series of paired sample t-tests were used to determine the effect of condition on gestures, vocalizations, barters, rump presents, and attention-getting behaviors, as well as the time to first response. McNemar tests were used to evaluate differences in the first response of subjects in the two conditions. Because fewer than 20 subjects exhibited an attention-getting behavior as their first response in only one of the two conditions, a binomial probability was calculated for this behavior.
Results
Descriptive statistics
There was considerable variation in the frequencies of each observed behavior both within each condition and between the two conditions. However, gestures and vocalizations were the most frequently observed behaviors in both conditions. There was also considerable variation in the number of chimpanzees contributing to the frequencies in each behavioral category. Table 1 shows the number of times each behavior occurred, as well as how many animals exhibited each behavior in both the tool and no-tool condition. Rump present and barters made up only 1% of total behaviors and were not included in subsequent analysis.
Table 1.
Frequencies of each behavior and the number of animals exhibiting each behavior
| Behavioral category | Testing conditions |
|||
|---|---|---|---|---|
| Experiment 1 (n=105) |
Experiment 2 (n64) |
|||
| Tool | No-tool | Toward | Away | |
| Gesture | ||||
| No. of occurrences | 129 | 43 | 124 | 42 |
| No. of chimpanzees | 55 | 24 | 35 | 15 |
| Vocalization | ||||
| No. of occurrences | 139 | 54 | 113 | 64 |
| No. of chimpanzees | 55 | 33 | 31 | 22 |
| Display | ||||
| No. of occurrences | 27 | 7 | 0 | 6 |
| No. of chimpanzees | 17 | 5 | 0 | 5 |
| Cage bang | ||||
| No. of occurrences | 45 | 24 | 60 | 58 |
| No. of chimpanzees | 17 | 14 | 15 | 15 |
| Spit | ||||
| No. of occurrences | 13 | 9 | 10 | 7 |
| No. of chimpanzees | 8 | 5 | 4 | 3 |
| Throw | ||||
| No. of occurrences | 12 | 9 | 7 | 3 |
| No. of chimpanzees | 7 | 6 | 4 | 1 |
| Clap | ||||
| No. of occurrences | 4 | 1 | 7 | 4 |
| No. of chimpanzees | 3 | 1 | 2 | 2 |
| Rump present | ||||
| No. of occurrences | 1 | 1 | 4 | 0 |
| No. of chimpanzees | 1 | 1 | 2 | 0 |
| Barter | ||||
| No. of occurrences | 3 | 2 | 4 | 1 |
| No. of chimpanzees | 3 | 2 | 4 | 1 |
Out of the 117 subjects tested, 105 were observed to exhibit at least one behavioral response in at least one of the two conditions. Of the remaining 12 subjects, there were 6 females and 6 males. Seven of these subjects were mother-reared, 1 was nursery-reared, and 4 were wild-caught. The 12 subjects that did not respond in either condition were excluded from the analysis of condition effects.
Analysis of condition effects
A series of paired t-tests revealed significant differences between conditions for the behaviors gesture, t(104) 4.282, P <0.001, vocalization, t(104)=3.549, P=0.001, and display, t(104)=2.704, P <0.01, as well as for the time to first response, t(104)=−4.635, P<0.001. No significant difference was found for attention-getting behaviors, t(104)=1.458, P=0.148. Figure 2 shows the mean number of gestures, vocalizations, displays, and attention-getting behaviors in the tool and no-tool conditions.
Fig. 2.
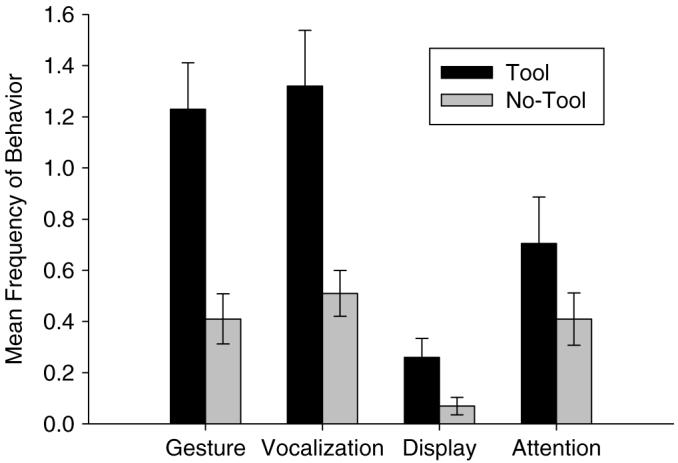
Mean frequencies (+SE) of gestures, vocalizations, displays, and attention-getting behaviors in the tool and no-tool conditions
Rearing and sex differences
Using a univariate ANOVA with the difference score as the dependent variable and sex and rearing as the fixed factors, a significant interaction was found between rearing and sex, F(2, 111)=4.926, P<0.01. Post-hoc analysis revealed that the difference scores of males and females only differed significantly for the nursery-reared animals, F(1, 35)=4.249, P<0.05. Table 2 shows the mean difference scores for males and females in each of the three rearing categories as well as post-hoc results for each rearing condition. The difference scores of individuals who were mother-reared, nursery-reared, or wild caught did not differ significantly across males, F(2, 52)=2.637, P=0.081, or females, F(2, 59)=3.120, P=0.052.
Table 2.
Mean difference scores for females and males by rearing history
| Rearing | Sex | Mean | Error | F | P |
|---|---|---|---|---|---|
| Mother | Females (n=23) | 1.435 | 0.812 | 2.718 | 0.107 |
| Males (n=19) | 3.211 | 0.894 | |||
| Nursery | Females (n=19) | 0.158 | 0.894 | 4.249 | 0.047* |
| Males (n=18) | 2.278 | 0.918 | |||
| Wild | Females (n=20) | 3.500 | 0.871 | 3.312 | 0.077 |
| Males (n=18) | 0.611 | 0.918 |
Analysis of first response data
In addition to the frequencies of each communicative behavior, we also compared the first communicative response between conditions. In these analyses, McNemar tests were used to compare occurrences of each behavioral category in the same subjects in the two conditions. Significant differences were found for gestures X2(1, n=117)=12.60, P<0.001 and no response X2(1, n=117)=18.62, P<0.001. Of the subjects that exhibited a gesture as their first response in only one of the two conditions, 33 gestured first only in the tool condition compared to 9 subjects that gestured first in the no-tool condition. In contrast, of the subjects that did not respond in only one of the two conditions, significantly more failed to respond when the experimenter did not have a tool (n=44) compared to when she had a tool (n=11). No significant differences were found for vocalizations, X2(1, n=117)=1.09, P=0.296 or for attention-getting behaviors (binomial probability, P=0.523).
Discussion
The results of experiment 1 are straightforward. Chimpanzees exhibit more communicative behaviors when a human experimenter possesses a desired tool than when they do not. Moreover, the first communicative response was more likely to be a manual gesture in the tool compared to the no-tool condition. The chimpanzees also communicated significantly faster in the tool than no-tool condition. Thus, chimpanzees do communicate with humans about objects other than food. In the wild, many studies have shown that chimpanzees use manual gestures, vocalizations, and other behaviors to communicate with each other in non-food related contexts (see Goodall 1986; Tomasello et al. 1985; De Waal 1988). However, in the captive setting, most studies of chimpanzee communication have been in the context of the subject interacting with a human experimenter in order to request food. The current study demonstrates that food is not a prerequisite for the production of the types of intra-species communicative behaviors reported previously in these captive great apes (e.g. Bard 1992; Call and Tomasello 1994; Leavens and Hopkins 1999).
Experiment 1 also revealed a sex by rearing interaction on the subjects' appropriate communicative responding (demonstrated by the difference scores). However, this finding is difficult to interpret because in our sample rearing is confounded by age. Wild-caught chimpanzees are substantially older than mother and nursery-reared animals. The mean age of a wild-caught animal in our sample is 33.68 years (±5.25SD) while the mean age for a mother-reared animal is 13.12 years (±5.55SD) and for a nursery-reared animal is 17.27 years (±7.21SD). Looking at Table 2, both mother and nursery-reared males have higher difference scores than mother and nursery-reared females while wild-caught males have lower difference scores than wild-caught females. It is difficult to know, based on our sample, if this effect is isolated to older males or if it is specific to wild-caught males since essentially these two groups are one in the same. Short of capturing young males from their native homes in Africa and bringing them into captivity (which these authors definitely do not endorse) this question will remain unanswered.
In experiment 2 we examined whether chimpanzees use different communicative signals as a function of the attentional status of a human that has a tool needed by the subject.
Experiment 2
Methods
Subjects
Twenty-seven chimpanzees from the Yerkes colony and 82 chimpanzees from the Bastrop colony were available for testing in experiment 2 including 58 females and 51 males. Of the 109 subjects, 49 were mother-reared, 23 were nursery-reared and 37 were wild-caught (see previous description). Subjects ranged in age from 6 to 46 years (mean±SD =22.95±10.67). As in experiment 1, all subjects were experienced with the honey dipping device used in this experiment.
Materials
The housing conditions of the subjects and the honey dipping device used were the same as in experiment 1. A Canon NTSC 2R20 digital video camcorder mounted on a Velbon CX200 tripod was used to record the behaviors of subjects in the away condition since real-time scoring by the experimenter was impossible for this condition (see the following Procedures section for a description of the away condition). Video clips were then captured using Roxio Videowave Movie Creator for Microsoft Windows and scored using Windows Media Player.
Procedure
The honey dipping device was placed and baited in the same manner as in experiment 1. The experimenter then left the testing area for a minimum of 60 s before returning to begin the trial. The first condition of experiment 2, referred to as the toward condition exactly replicated the tool condition of the first experiment. The experimenter was positioned in front of and facing the focal subject's home cage holding a set of tools in her hand. The second condition, referred to as the away condition sought to vary the attentional state of the human experimenter. In this condition, a video camera was set up to record the behaviors of the subjects during the 5-min observation period. The experimenter approached the cage with a handful of reeds and made sure the subject was on camera before turning on the camera and immediately sitting approximately 1 m from the mesh with her back turned towards the subject. The experimenter held the reeds by her side so that the tools were still in view of the chimpanzee, but she never made eye contact or turned to face the subject for the duration of the 5-min observation period. The same behavioral ethogram used in experiment 1 was used in experiment 2. Behaviors were scored real-time by the experimenter in the toward condition but were scored from video clips for the away condition. For both conditions, the scorer noted the time elapsed from the onset of the trial to the first behavioral response by the subject. At the end of each trial, all subjects were given a tool and allowed to use the honey dipping device. Order of presentation of the toward and away conditions were counterbalanced across cages and each subject received only one trial in each condition.
Data analysis
As in experiment 1, the behaviors cage bang, spit, clap, and throw were categorized together as attention-getting behaviors for the purpose of statistical analysis. As in experiment 1, the behaviors rump present and barter were observed so infrequently they were excluded from subsequent analyses. Paired sample t-tests were used to determine the effect of condition on gestures, vocalizations, displays, and attention-getting behaviors, as well as on the time to first response. A repeated measures analysis of variance (ANOVA) was used to test the effects of sex and rearing on the total number of responses across conditions while controlling for age. A McNemar test was used to evaluate the difference in the number of subjects that did not respond in only one of the two conditions. Because fewer than 20 subjects gestured, vocalized, displayed, or exhibited an attention-getting behavior as their first response in only one of the two conditions, binomial probabilities were calculated for these behaviors.
Results
Descriptive statistics
The distribution of each behavior of interest was similar to experiment 1 with gestures and vocalizations being the most frequently observed behaviors, closely followed by cage bangs. Table 1 shows the frequency of each behavior, as well as the number of animals that exhibited each behavior across conditions. Of the 103 subjects tested, 64 subjects exhibited at least one behavioral response during one of the two conditions. Therefore, with the exception of first response data analysis, subsequent analyses were restricted to this subset of individuals. This subset of subjects was made up of 34 females and 30 males. With respect to rearing history, there were 25 mother-reared, 17 nursery-reared, and 22 wild-caught animals.
An important difference to note between experiments 1 and 2 is the proportion of animals who did not respond in either condition. In the first experiment, 89.74% of subjects responded while only 62.13% of subjects responded in experiment 2. Possible explanations for this difference are examined in the discussion section below.
Analysis of condition effects
First, a paired samples t-test revealed that chimpanzees produced more overall communicative behaviors in the toward than the away condition, t(63)=2.60, P<0.05. Furthermore, additional paired samples t-tests revealed significant differences between the toward and away conditions for the specific behaviors gesture, t(63)=3.14, P<0.01, vocalization t(63)=2.39, P<0.05 and display, t(63)=−2.18, P<0.05, as well as for the time to first response, t(63)=−3.51, P=0.001. No significant differences were found for attention-getting behaviors, t(63)=0.29, P=0.775. Figure 3 shows the mean number of gestures, vocalizations, displays, and attention-getting behaviors for the toward and away conditions.
Fig. 3.
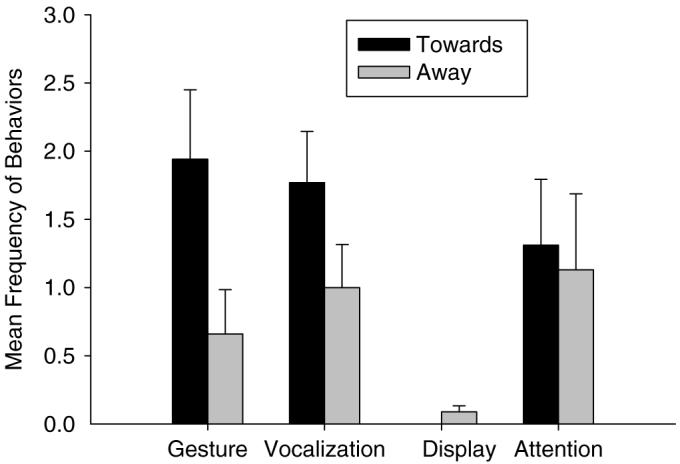
Mean frequencies (+SE) of gestures, vocalizations, displays, and attention-getting behaviors in the toward and away conditions
Of the 64 subjects that responded in at least one of the two conditions, 29 communicated in both conditions, 28 communicated in the toward but not the away condition, and 7 communicated in the away but not the toward condition. A Chi-square test revealed that subjects were not equally distributed in the three categories of behavioral responses, X2(2, n=64)=14.47, P=0.001. Further analysis revealed no significant difference in the distribution of subjects that communicated in both conditions from those that communicated in only the toward condition, X2(1, n=57)=0.02, P=0.895. However, the number of subjects responding only in the away condition was significantly less than the number of subjects responding in both conditions, X2(1, n=36)=13.44, P<0.001 and the number of subjects responding only in the toward condition, X2(1, n=35)=12.60, P<0.001
Rearing, sex, and age effects
A repeated measures ANOVA with the total number of communicative responses in each condition serving as the dependent variables, sex and rearing as the fixed variables, and age as the covariate failed to reveal any significant main effects or interactions.
Analysis of first response
McNemar tests and binomial probabilities were used to evaluate whether or not there was an effect of condition on the first behavioral response produced by the subjects. Significant differences between conditions were found for gestures, binomial probability, P=0.031 and for no response X2(1, n=103)=11.429, P=0.001. Of the subjects that gestured first in only one of the two experiments, 14 first responded with a gesture in the toward condition compared to only 4 that responded first with a gesture in the away condition. In addition, of the chimpanzees that did not respond in only one of the two conditions, 28 failed to respond in the away condition compared to only 7 in the toward condition. No significant differences were found for vocalizations, (binomial probability, P=0.078), displays, (binomial probability, P=1.00), or attention-getting behaviors, (binomial probability, P=0.607).
Discussion
The results of experiment 2 show that chimpanzees attempt to communicate more with an experimenter who is looking at them than with an experimenter who has her back turned toward them. More specifically, subjects in this study gestured and vocalized more when the experimenter was facing them but displayed more when the experimenter had her back turned toward them. This last finding may be indicative of frustration on the part of the chimpanzee when the human experimenter did not turn to look at the subject since a display is an emotional response to being upset. Subjects also responded more quickly in the toward condition than in the away condition. Further analysis revealed that of the 64 subjects who communicated in the second experiment, only 7 communicated in the away condition but not in the toward condition. In addition, the first response data showed that the chimpanzees were more likely to use a gesture (a visual communicative behavior) as their first communicative behavior in the toward condition but more likely to not respond at all in the away condition. This data demonstrates that chimpanzees differentially respond based on the attentional state of a human experimenter and is consistent with other reports that apes are sensitive to the attentional status of a human communicative partner (Hostetter et al. 2001; Krause and Fouts 1997; Povinelli and Eddy 1996).
The fact that so many subjects in experiment 2 did not respond at all compared to experiment 1 is interesting. The most likely explanation for this difference is that the subjects learned the experimental paradigm during the two trials of the first experiment. Recall that the experimental design included a lengthy 5-min observation period in which subjects did not receive a tool no matter what communicative responses they produced and did receive a tool at the end of the 5-min observation period even if they had stopped communicating with the experimenter. Subjects quite possibly learned that communicating did not get them a tool and that if they simply waited 5 min a tool would be given to them regardless of their behaviors. Therefore, during experiment 2 many subjects may have decided it was more efficient to look for a tool in their enclosure or simply wait for the end of the 5 min when they knew they would receive a tool than it was to attempt to communicate with an unresponsive experimenter.
Experiment 3 aimed to further explore the intentional and goal-directed nature of chimpanzees' communication regarding tools by recording their response to receiving an ineffective tool. In order to reduce the possible confound of learning on the subjects willingness to communicate, several months separated data collection for the first two experiments from data collection in the third experiment. In addition, the observation time was reduced from 5 min to 3 min and the experimenter was located directly in front of the tool use device to make communicating with the experimenter easier for the subjects.
Experiment 3
Methods
Subjects
A small cohort of 35 Yerkes chimpanzees were tested in the third experimental paradigm. Of the 35 subjects, 14 were males and 21 were females. Subjects ranged in age from 8 to 46 years (mean±SD=21.23±10.37). Ten subjects were mother-reared, 18 were nursery-reared, and 7 were wild-caught. As in the first two experiments, all of the subjects were experienced tool users with the honey dipping device.
Materials
The housing conditions of the subjects and the honey dipping device used were identical to those used in the first two experiments. All scoring was done real-time without the use of video. Two lengths of reed sticks were used for the tools in experiment 3. “Effective tools” were identical to those used in experiments 1 and 2 and measured approximately 18 cm in length. “Ineffective tools” were made of the same material but were cut to measure only 3–4 cm. in length rendering them useless for retrieving food from the honey dipping device.
Procedure
The setup of the third experiment was very similar to that of experiments 1 and 2. One main difference was the location of the experimenter in relation to the tool use device. Since the first two experiments clearly established that chimpanzees will communicate with a human experimenter about tools that are separated by some distance from the actual feeding device, the experimenter in experiment 3 located herself approximately 1 m from the cage mesh in front of the honey dipping device rather than in front of an adjacent cage.
The design of the third experiment was very straightforward. Once the honey dipping device had been baited, the experimenter waited a minimum of 60 s before approaching the cage with a handful of reeds and sitting down facing the subject approximately 1 m from the cage front. The experimenter then recorded all communicative behaviors (gesture, vocalization, spit, clap, cage bang, rump present, display, barter, and throw) of the focal subject for a period of 3 min. This observation period is referred to as the pre-test condition. The subject then experienced one of two experimental conditions. In one condition the subject was given an effective tool (effective tool condition, ETC). In the other condition the subject was given an ineffective tool (ineffective tool condition, ITC). As described above, the ineffective tools were too short to be used to retrieve honey from the device. For both the ETC and the ITC the experimenter recorded all communicative behaviors for 3 min following the delivery of the tool. The ETC and the ITC were always preceded by a 3-min pre-test. Each subject received one trial of both the ETC and the ITC. The order of presentation of the two conditions was counterbalanced across subjects.
Data analysis
Paired samples t-tests were used to assess any possible differences in communicative behavior as a result of having received an effective tool versus an ineffective tool. The time elapsed to the first communicative response and the total number of communicative behaviors exhibited in a given condition were the dependent variables of interest. All communicative behaviors were collapsed because there was no theoretical expectation of differing modes of communication in the two conditions. Alpha was set at P<0.5 for all analyses.
Descriptive statistics
All but 4 of the 35 subjects (2 males and 2 females) responded in at least one of the four conditions. This represents an 88.57% participation rate which approximates that of experiment 1 (89.74%) so it appears that the elapsed time between experiment 3 and the first two experiments in combination with the modifications to the experimental design were sufficient to eliminate any effects of the initial experiments which may have precluded subjects from responding. As in the previous two experiments, gestures and vocalizations were the most frequently observed behaviors across conditions.
Analysis of the pre-tests
To ensure that the experimenter was in no way cueing the animals as to which condition would follow the pre-test, a paired samples t-test was performed on the time to first response during the pre-test of the effective and ineffective tool conditions. No significant difference was found between the two pre-tests, t(34)=−0.46, P=0.646. To further address this issue, a paired samples t-test was used to compare the total number of communicative behaviors during the pre-tests of the effective and ineffective tool conditions. Again, no significant difference was found, t(34)=1.91, P=0.065.
Analysis of condition effects
In contrast to the above results, a paired samples t-test examining the difference in the time to first response following the receipt of an effective tool versus an ineffective tool revealed that subjects communicated with the experimenter significantly faster after receiving the ineffective tool than after receiving the effective tool, t(34)=2.24, P<0.05. In addition, the chimpanzees exhibited significantly more communicative behaviors following the receipt of an ineffective tool than after receiving an effective tool, t(34)=−3.070, P<0.01. The mean time to first response for each condition can be seen in Fig. 4. The mean number of communicative behaviors for each condition can be seen in Fig. 5.
Fig. 4.
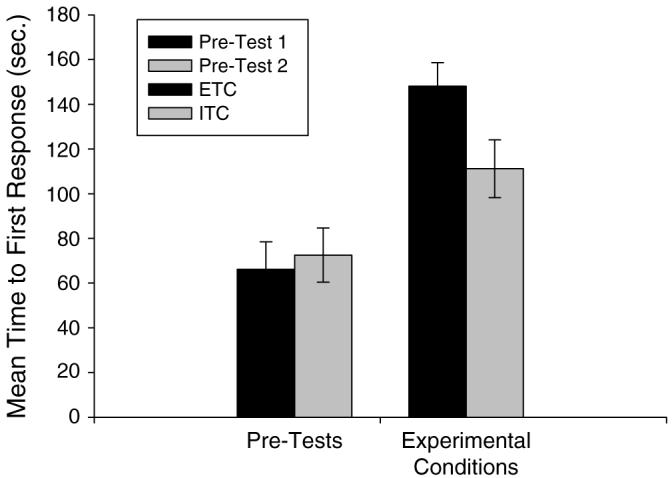
Mean time to the first communicative response for both pre-tests (1, 2), the effective tool condition (ETC), and the ineffective tool condition (ITC)
Fig. 5.
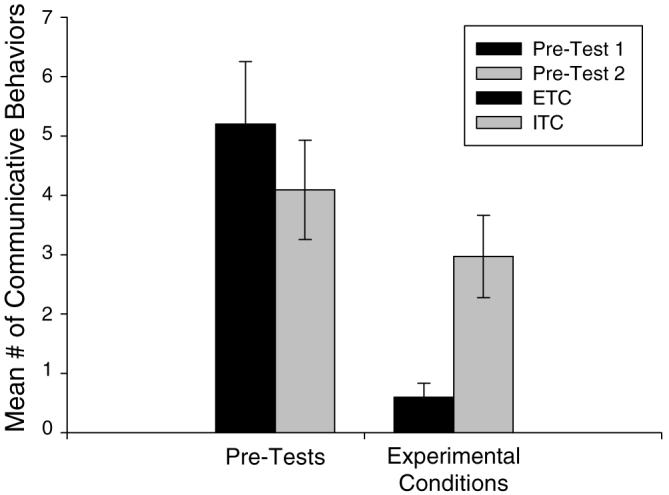
Mean number of communicative behaviors exhibited in both pre-tests (1, 2), the effective tool condition (ETC), and the ineffective tool condition (ITC)
Discussion
The results of experiment 3 are straightforward. The chimpanzees in this study continued to communicate with the experimenter after being given an ineffective tool much more so than after being given an effective tool. They were also faster to communicate with the experimenter in the IFC than in the EFC. This indicates that the behaviors observed here (manual gestures, vocalizations, etc.) were about a specific object, not just a generalized response to the experimenter.
General discussion
The results of experiment 1 are clear, chimpanzees communicate about objects other than food. Although food has been the primary focus of previous studies on manual gestures in great apes (e.g. Bard 1992; Call and Tomasello 1994; Leavens and Hopkins 1999), the current study demonstrates that food is not a prerequisite for the production of gestures and other communicative signals (i.e. vocalizations). The results of experiment 2 further demonstrate that the chimpanzees are sensitive to the presence of an attentive audience in their communicative behaviors and can volitionally produce or inhibit their signals depending on the attentional state of a human experimenter.
The results of experiment 2 suggest that chimpanzees do respond differentially based on the attentional status of a human communicative partner. First, chimpanzees in this study responded faster when the experimenter was facing the subject than when the experimenter had her back turned. Second, chimpanzees gestured and vocalized more frequently in the toward condition than in the away condition but displayed more frequently in the away condition than in the toward condition. In addition, subjects were more likely to respond with a gesture first when the experimenter was facing them and more likely to not respond at all when the experimenter was looking away. Overall, subjects were more willing to communicate with an attentive human than with a human that had her back turned toward them. Although these results do not fully support the findings by Hostetter et al. (2001) in that vocal signaling decreased rather than increased when the audience was inattentive, there are three important factors to consider.
First, chimpanzees in the current study communicated significantly more often when the experimenter was facing them than when she was not. This indicates that the subjects were sensitive to the attentional state of the experimenter but that perhaps the away condition did not provide a salient enough situation to elicit many communicative responses. Recall that the current experimental design had the experimenter located some distance from the honey dipping device so that subjects had to move through at least one enclosure or across the distance of a large enclosure in order to reach the experimenter. In addition, the experimenter was holding tools, not food as in the Hostetter et al. (2001) study. Perhaps moving a large distance away from a food source to an inattentive experimenter holding tools did not create a motivating enough situation to elicit communicative responses. The fact that fewer subjects were willing to communicate in either condition of the second experiment than were willing in the first experiment suggests that perhaps their overall lack of communication in the away condition was more pronounced than any possible differences in the particular types of behaviors elicited by the two conditions.
The second factor is one of methodology. Hostetter et al. (2001) recorded behavior for a period of 60 s, whereas in the current study, a trial lasted for 5 min. During a trial, the experimenter was unresponsive to any communicative attempt by the chimpanzee. The longer trial time may, in essence, have created a situation in which there was a seemingly inattentive audience despite the fact that the experimenter was facing the subject. In a study done by Leavens et al. (2005), chimpanzees were given a choice between a highly desirable food item (a banana) and a less desirable food item (chow). In one condition the subject was given the banana, in a second condition the subject was given half of a banana while the experimenter placed the other half in his or her pocket, and in a third condition the subject was given the chow. The chimpanzees were shown to make attempts to repair their failed communicative bid after being given less than the whole banana by elaborating on the types of communicative signals they used in the half banana and chow conditions. In experiment 2, the chimpanzees may have been elaborating their communicative signals in the toward condition to the point that any differential use of their signals were masked by the extended observation period.
Lastly, in the Hostetter et al. (2001) study, the human holding the food was positioned in front of the subject in one cage. In contrast, in experiment 2 of the current study, recall that the tool device was in a different cage relative to the position of the human. Thus, the subjects had to not only adjust their communicative signals but also move to the position of the human in order to produce the communicative behavior (see also Liebel et al. 2004). Thus, the subjects had to be highly motivated in this study compared to the chimpanzees in the study by Hostetter et al. (2001).
Experiment 3 added to this body of data by addressing the goal-directed and persistent nature of chimpanzee communication. The chimpanzees recognized that the tool they were given in the ineffective tool condition could not serve the purpose of extracting honey from the device and they therefore continued trying to communicate to the experimenter. It is clear that the chimpanzees in experiment 3 were not simply trying to get a generalized response from the experimenter of giving them something but that they were trying to request something very specific.
In summary, the results of this study indicate that chimpanzee's gesture for non-food items that have an instrumental function for them. Our results suggest that the chimpanzees' gestural and vocal signals (at least those used in this study) are not simply a result of emotional arousal induced by the presence of food but rather serve an intentional, communicative function (Leavens et al. 1996; Leavens and Hopkins 1998; Tomasello et al. 1985). Furthermore, chimpanzees can modulate the types of behaviors they use based on the attentional state of their human communicative partner (Tomasello et al. 1994, Tomasello and Camaioni 1997; Hostetter et al. 2001). Lastly, extended human contact or enculturation does not appear to be a necessary condition for the expression of intentional and referential communicative signaling by chimpanzees (Leavens and Hopkins 1998, 1999; Leavens et al. 2004). It is still unknown whether great apes use manual gestures or other communicative signals in a declarative, rather than imperative, context. In this and many other studies, the chimpanzees were requesting rather than gesturing simply to make a declarative statement about something in their environment. Some have suggested that apes and humans are distinguished by their use of imperative contrasted with declarative signaling, and future research should focus on addressing this important dimension of communication.
Acknowledgments
This work was supported in part by NIH grants RR-00165, U42-RR-15090, NS-42867, NS-36605 and HD-38051. We thank the care staff from each facility for assistance in data collection. The YRPRC and UTMDACC facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. American Psychological Association guidelines for the care and use of animals were adhered to during all aspects of this study
References
- Bard K. Intentional behavior and intentional communication in young free-ranging orangutans. Child Dev. 1992;63:1186–1197. [PubMed] [Google Scholar]
- Call J, Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus) J Comp Psychol. 1994;108:307–317. doi: 10.1037/0735-7036.108.4.307. [DOI] [PubMed] [Google Scholar]
- De Waal F. The communicative repertoire of captive bonobos, Pan paniscus, compared to that of chimpanzees. Behaviour. 1988;106:183–251. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns in adaptation. Harvard University Press; Cambridge, Mass.: 1986. [Google Scholar]
- Harding CG, Golinkoff RM. The origins of intentional vocalizations in prelinguistic infants. Child Dev. 1979;50:33–40. [PubMed] [Google Scholar]
- Hostetter AB, Cantero M, Hopkins WD. Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to the attentional status of a human (Homo sapiens) J Comp Psychol. 2001;115:337–343. doi: 10.1037//0735-7036.115.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause MA, Fouts RS. Chimpanzee (Pan troglodytes) pointing: hand shapes, accuracy, and the role of eye gaze. J Comp Psychol. 1997;111:330–336. doi: 10.1037/0735-7036.111.4.330. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees: a cross-sectional study of the use of referential gestures. Dev Psychol. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. The whole-hand point: the structure and function of pointing from a comparative perspective. J Comp Psychol. 1999;113:417–425. doi: 10.1037/0735-7036.113.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) J Comp Psychol. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Thomas RK. Referential communication by Chimpanzees (Pan troglodytes) J Comp Psychol. 2004;118:48–57. doi: 10.1037/0735-7036.118.1.48. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Russell JL, Hopkins WD. Intentionality as measured in the persistence and elaboration of communication by chimpanzees (Pan troglodytes) Child Dev. 2005;76:291–306. doi: 10.1111/j.1467-8624.2005.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebal K, Pika S, Call J, Tomasello M. To move or not to move: how apes adjust to the attentional state of others. Interact Stud. 2004;5:199–219. [Google Scholar]
- Pika S, Liebal K, Tomasello M. Gestural communication in young gorillas (Gorilla gorilla): gestural repertoire, learning, and use. Am J Primatol. 2003;60:95–111. doi: 10.1002/ajp.10097. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. Factors influencing young chimpanzees' (Pan troglodytes) recognition of attention. J Comp Psychol. 1996;110:336–345. doi: 10.1037/0735-7036.110.4.336. [DOI] [PubMed] [Google Scholar]
- Schaller GB. The mountain gorillas. Ecology and behavior. University of Chicago Press; Chicago: 1963. [Google Scholar]
- Tanner JE, Byrne R. Representation of action through iconic gesture in captive lowland gorilla. Curr Anthropol. 1996;37:162–173. [Google Scholar]
- Tanner JE, Byrne R. The development of spontaneous gestural communication in a group of zoo-living lowland gorillas. In: Parker T, Milks S, Mitchell R, editors. The mentalities of gorillas and orangutans, comparative perspectives. Cambridge University Press; Cambridge, Mass.: 1999. pp. 211–239. [Google Scholar]
- Tomasello M, George BL, Kruger AC, Farrar MJ, Evans A. The development of gestural communication in young chimpanzees. J Hum Evol. 1985;14:175–186. [Google Scholar]
- Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and use of gestural signals by young chimpanzees: a trans-generational study. Primates. 1994;37:137–154. [Google Scholar]
- Tomasello M, Camaioni L. A comparison of the gestural communication of apes and human infants. Human Dev. 1997;40:7–24. [Google Scholar]


