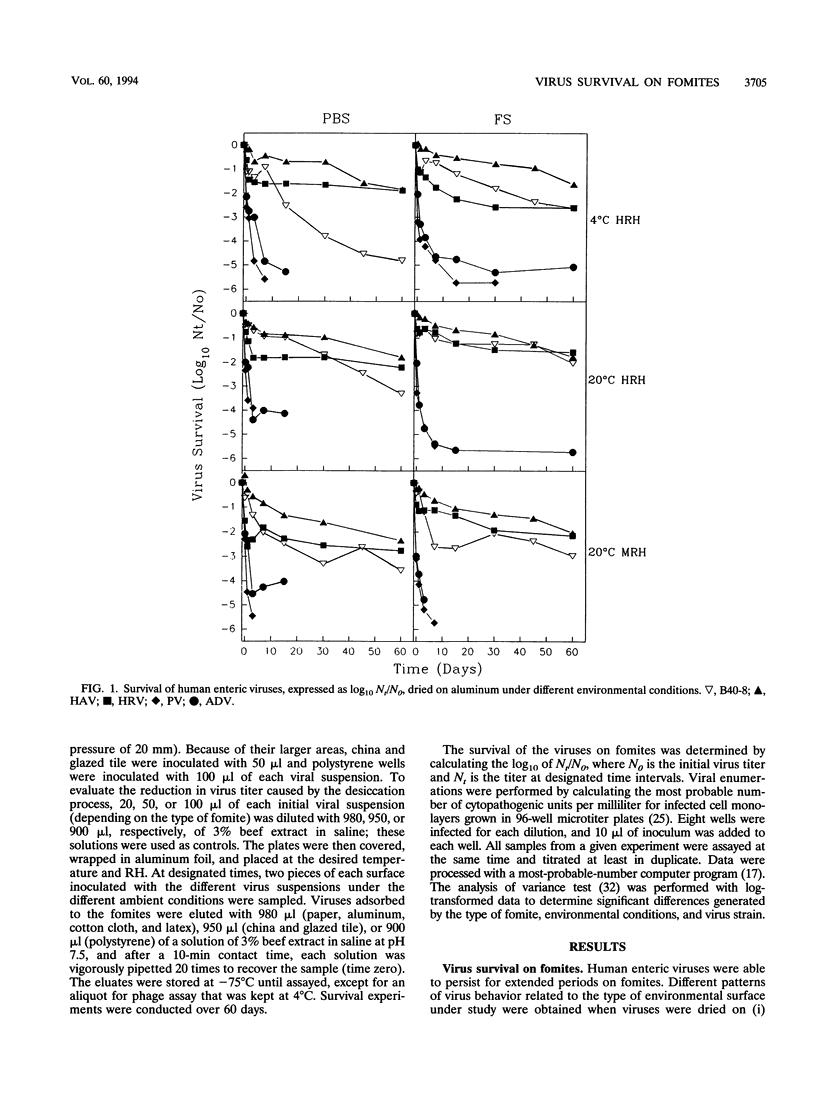
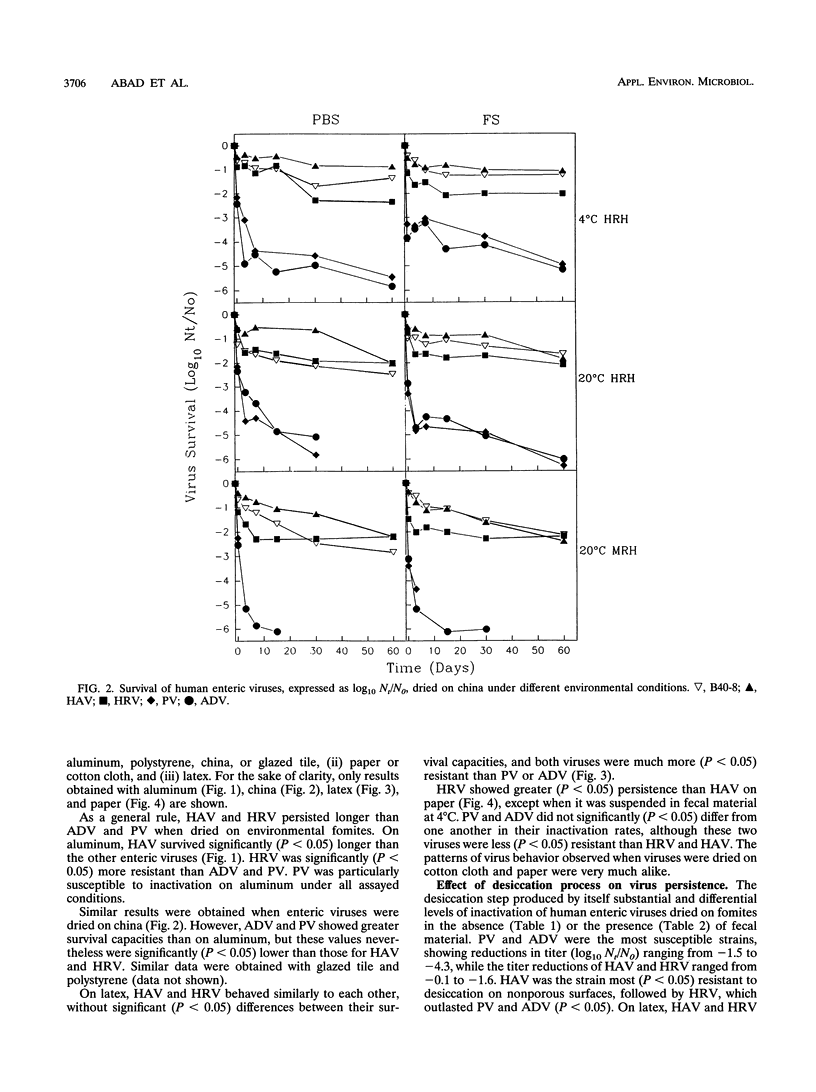
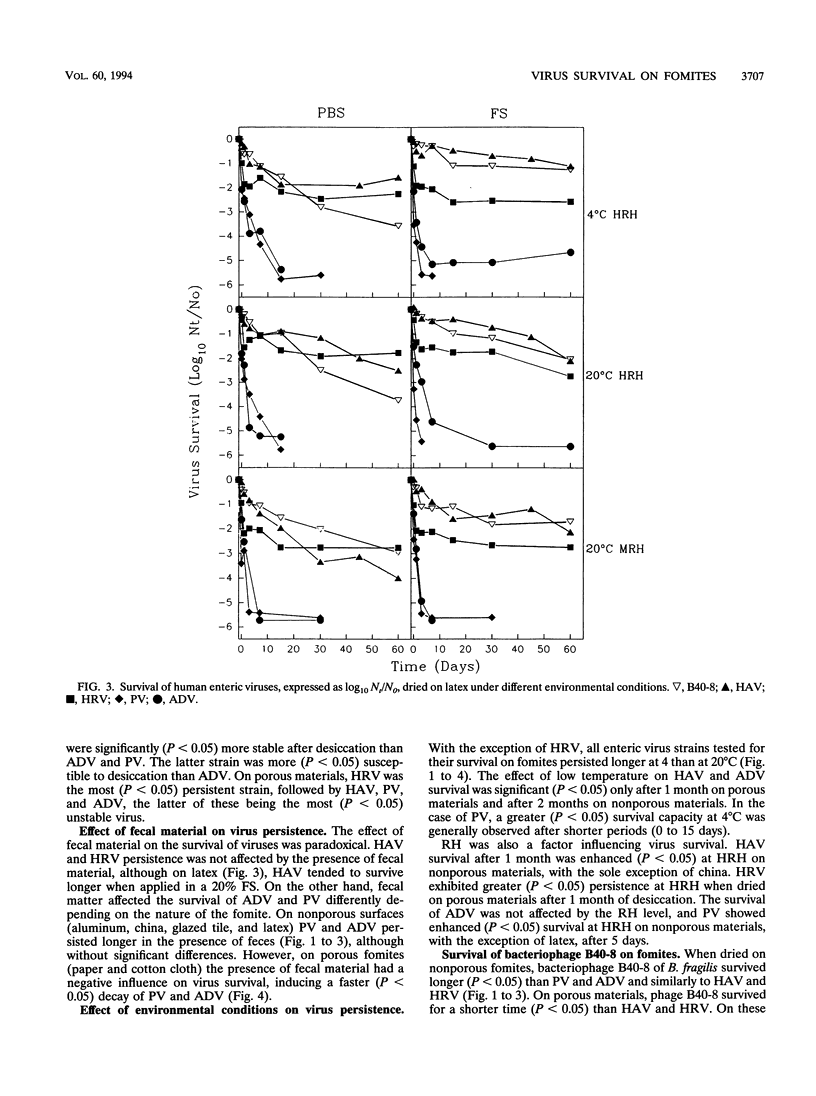
Abstract
The survival of human enteric viruses on several porous (paper and cotton cloth) and nonporous (aluminum, china, glazed tile, latex, and polystyrene) environmental surfaces has been evaluated. Viruses persisted for extended periods on several types of materials commonly found in institutions and domestic environments. The stability of the viruses was generally influenced by environmental factors such as relative humidity (RH), temperature, and the type of surface contaminated. Overall, hepatitis A virus (HAV) and human rotavirus (HRV) were more resistant to inactivation than enteric adenovirus (ADV) and poliovirus (PV). The resistance to the desiccation step appears to be of major significance in determining the survival of a virus dried on fomites. ADV and PV showed a pronounced decrease in titer at this stage, whereas HAV and HRV displayed little decay at the desiccation step. HAV and HRV persistence was not affected by the presence of fecal material. On nonporous surfaces, PV and ADV persisted better in the presence of feces. However, on porous fomites the presence of fecal material had a negative influence on the survival of PV and ADV. Except for HRV, greater virus survival was observed at 4 degrees than at 20 degrees C. PV and HAV survival was enhanced at high RH; the survival of the latter was enhanced at least for nonporous materials. When dried on porous materials, HRV also exhibited greater persistence at high RH. The survival of ADV was not affected by RH. The validity of using bacteriophages of Bacteroides fragilis as indicators of human viruses dried on fomites was evaluated.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCKLAND F. E., TYRRELL D. A. Loss of infectivity on drying various viruses. Nature. 1962 Sep 15;195:1063–1064. doi: 10.1038/1951063a0. [DOI] [PubMed] [Google Scholar]
- Bern C., Martines J., de Zoysa I., Glass R. I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ. 1992;70(6):705–714. [PMC free article] [PubMed] [Google Scholar]
- Bosch A., Pinto R. M., Jofre J. Non-seasonal distribution of rotavirus in Barcelona raw sewage. Zentralbl Bakteriol Mikrobiol Hyg B. 1988 Jun;186(3):273–277. [PubMed] [Google Scholar]
- Butz A. M., Fosarelli P., Dick J., Cusack T., Yolken R. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993 Aug;92(2):202–205. [PubMed] [Google Scholar]
- Dickinson C. J. Hepatitis A--new information on an old virus. Hepatology. 1992 Oct;16(4):1099–1101. doi: 10.1002/hep.1840160442. [DOI] [PubMed] [Google Scholar]
- ENRIGHT J. R. The epidemiology of paralytic poliomyelitis in Hawaii. Hawaii Med J. 1954 May-Jun;13(5):350–354. [PubMed] [Google Scholar]
- Flewett T. H., Beards G. M., Brown D. W., Sanders R. C. The diagnostic gap in diarrhoeal aetiology. Ciba Found Symp. 1987;128:238–249. doi: 10.1002/9780470513460.ch14. [DOI] [PubMed] [Google Scholar]
- Gerba C. P. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol. 1984;30:133–168. doi: 10.1016/s0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- Halvorsrud J., Orstavik I. An epidemic of rotavirus-associated gastroenteritis in a nursing home for the elderly. Scand J Infect Dis. 1980;12(3):161–164. doi: 10.3109/inf.1980.12.issue-3.01. [DOI] [PubMed] [Google Scholar]
- Hasegawa A., Matsuno S., Inouye S., Kono R., Tsurukubo Y., Mukoyama A., Saito Y. Isolation of human rotaviruses in primary cultures of monkey kidney cells. J Clin Microbiol. 1982 Aug;16(2):387–390. doi: 10.1128/jcm.16.2.387-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A. H., van Olphen M., Drost Y. C. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl Environ Microbiol. 1993 Sep;59(9):2956–2962. doi: 10.1128/aem.59.9.2956-2962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejkal T. W., Smith E. M., Gerba C. P. Seasonal occurrence of rotavirus in sewage. Appl Environ Microbiol. 1984 Mar;47(3):588–590. doi: 10.1128/aem.47.3.588-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswick B. H., Pickering L. K., DuPont H. L., Woodward W. E. Survival and detection of rotaviruses on environmental surfaces in day care centers. Appl Environ Microbiol. 1983 Oct;46(4):813–816. doi: 10.1128/aem.46.4.813-816.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985 Oct 24;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- Levy B. S., Fontaine R. E., Smith C. A., Brinda J., Hirman G., Nelson D. B., Johnson P. M., Larson O. A large food-borne outbreak of hepatitis A. Possible transmission via oropharyngeal secretions. JAMA. 1975 Oct 20;234(3):289–294. [PubMed] [Google Scholar]
- Mbithi J. N., Springthorpe V. S., Sattar S. A. Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1991 May;57(5):1394–1399. doi: 10.1128/aem.57.5.1394-1399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Moe K., Shirley J. A. The effects of relative humidity and temperature on the survival of human rotavirus in faeces. Arch Virol. 1982;72(3):179–186. doi: 10.1007/BF01348963. [DOI] [PubMed] [Google Scholar]
- Moore M. Centers for Disease Control. Enteroviral disease in the United States, 1970-1979. J Infect Dis. 1982 Jul;146(1):103–108. doi: 10.1093/infdis/146.1.103. [DOI] [PubMed] [Google Scholar]
- Rochi G. U., Vella S., Resta S., Cochi S., Donelli G., Tangucci F., Menichella D., Varveri A., Inglese R. Outbreak of rotavirus gastroenteritis among premature infants. Br Med J (Clin Res Ed) 1981 Oct 3;283(6296):886–886. doi: 10.1136/bmj.283.6296.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder R. W., McGowan J. E., Hatch M. H., Palmer E. L. Reovirus-like agent as a cause of nosocomial diarrhea in infants. J Pediatr. 1977 May;90(5):698–702. doi: 10.1016/s0022-3476(77)81230-4. [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Dimock K. D., Ansari S. A., Springthorpe V. S. Spread of acute hemorrhagic conjunctivitis due to enterovirus-70: effect of air temperature and relative humidity on virus survival on fomites. J Med Virol. 1988 Jul;25(3):289–296. doi: 10.1002/jmv.1890250306. [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Lloyd-Evans N., Springthorpe V. S., Nair R. C. Institutional outbreaks of rotavirus diarrhoea: potential role of fomites and environmental surfaces as vehicles for virus transmission. J Hyg (Lond) 1986 Apr;96(2):277–289. doi: 10.1017/s0022172400066055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera C., Araujo R., Michel T., Jofre J. Culture and decontamination methods affecting enumeration of phages infecting Bacteroides fragilis in sewage. Appl Environ Microbiol. 1992 Aug;58(8):2670–2673. doi: 10.1128/aem.58.8.2670-2673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera C., Jofre J. Bacteriophages active against Bacteroides fragilis in sewage-polluted waters. Appl Environ Microbiol. 1987 Jul;53(7):1632–1637. doi: 10.1128/aem.53.7.1632-1637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



