Abstract
Detection of genetic diseases before implantation for couples at risk helps ensure healthy children, but testing for aneuploidy does not improve the chances of live birth in normal infertile women, say Peter Braude and Frances Flinter
A randomised trial in the New England Journal of Medicine has rekindled the acrimonious debate about the efficacy and appropriateness of testing for chromosomal imbalance (aneuploidy) before implantation in older infertile women having in vitro fertilisation.1 These women have such a poor prognosis of having a child by in vitro fertilisation that many will latch on to any promise that might improve their odds. This is the second randomised trial that shows no benefit from preimplantation genetic screening, yet advocates are unwilling to accept the findings. We examine the place of genetic testing of embryos in modern medical practice and possible future uses.
Preimplantation diagnosis
Preimplantation genetic diagnosis (PGD) was developed as an alternative to prenatal diagnosis and possible termination of an affected pregnancy for couples at risk of passing on a serious genetic disease to their children.2 3 It has an important place in preventing transmission of inherited conditions where the child has a high risk of dying early (such as spinal muscular atrophy),4 of severe mental or physical disability (such as unbalanced chromosome translocations),5 or of diseases such as Duchenne muscular dystrophy or cystic fibrosis that develop in childhood and shorten lifespan. In some cases the effect of the disease is so severe that it results in repeated early miscarriage (chromosome imbalance) or later fetal, neonatal, or infant death. Each of these conditions can be detected before implantation, provided the mutation within the relevant gene is known, the chromosome carrying the gene can be tracked through the family tree, or the specific chromosomal rearrangement has been identified.3
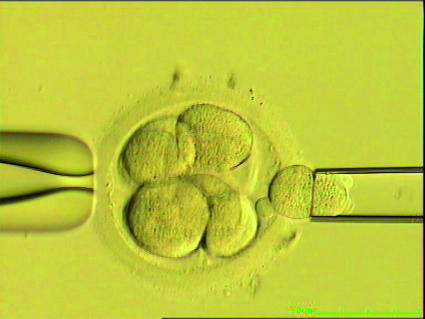
The technique was developed in the United Kingdom in the late 1980s and first used to avoid transmission of adrenoleucodystrophy and X linked mental retardation.6 Embryos were generated in vitro from couples who were generally fertile but had an affected child or a family history suggesting they were at high risk of an affected child. DNA was extracted from the single cell, amplified by the polymerase chain reaction, and tested for the specific mutation or, in the case of a sex linked disease, the presence of a Y chromosome associated genetic sequence (fig 1).6
Fig 1 Single blastomere being removed from 8 cell, day 3 embryo for preimplantation genetic diagnosis
The development of fluorescence in situ hybridisation (FISH),7 allowed specific chromosomes to be identified under the microscope, making sex selection simpler,8 and also enabled identification of embryos carrying unbalanced forms of translocations and other chromosome rearrangements.5.
The latest development in preimplantation genetic diagnosis is embryo haplotyping.9 This process allows the identification of a chromosome in the embryo that is likely to be carrying an inherited disorder through knowledge of the pattern of closely linked markers in an affected child or other family members. The main advantage of embryo haplotyping is that it does not require precise details of the mutation to be known, only which gene is implicated and the pattern of its inheritance in the family. This makes the development of a specific test for a disease faster and the diagnosis from a single cell biopsy more secure. It has superseded sex selection for couples at risk of having a son with a sex linked disorder because unaffected male embryos can be identified easily and considered for transfer.9
Despite its use for over 15 years, relatively few centres offer preimplantation diagnosis,10 and many of these send samples away for analysis rather than testing themselves. In the UK, there are now four centres with their own laboratories in London, one in Nottingham, and one in Glasgow, each doing 5-150 cases a year.
Success of preimplantation diagnosis
European data show that the chance of a successful outcome after preimplantation diagnosis depends on the type of genetic condition—whether it is autosomal recessive or dominant, sex linked, or a chromosome rearrangement (table 1).10 This is because the proportion of embryos likely to be unaffected varies with the inheritance of the disorder
Pregnancy rates after preimplantation diagnosis in 43 European centres related to type of genetic condition
| Condition | No of pregnancies/oocyte retrieved (%) | No of pregnancies/embryos transferred (%) |
|---|---|---|
| Chromosomal rearrangements* | 62/404 (15) | 62/234 (26) |
| Autosomal dominant disorders† | 88/517 (17) | 88/385 (23) |
| Sex linked disorders‡ | 120/635 (19) | 120/483 (25) |
| Autosomal recessive disorders§ | 161/669 (24) | 161/561 (29) |
*Reciprocal and robertsonian translocations.
†Myotonic dystrophy, amyloid polyneuropathy, adenomatous polyposis coli, Charcot Marie Tooth disease, achondroplasia, Marfan syndrome.
‡Duchenne muscular dystrophy, haemophilia, fragile X syndrome, etc.
§Cystic fibrosis, β thalassaemia, sickle cell anaemia, spinal muscular atrophy.
Since most couples who have preimplantation diagnosis are fertile, it had been thought that the pregnancy rates would be higher than when in vitro fertilisation is used for infertility. However, that assumption has not been substantiated. This is not really surprising, since not only do the embryos available for transfer have to survive the biopsy with good morphology but they must also be free of the genetic disorder. This requirement for the coincidence of two factors reduces the number of embryos suitable for transfer, and in many cases there may be none.
Success is most strongly related to the number of embryos available for biopsy, which in turn is related to the number of good quality eggs obtained after gonadotrophin stimulation, and this declines as the woman gets older. If suitable embryos are available for transfer, clinical pregnancy rates are around 25% regardless of the pattern of inheritance, although some clinics report higher successes than this.11 Data are not yet available on the number of live births per cycle attempted, which would take into account the likelihood that because of age or other factors, patients may not even get to egg collection.11
Preimplantation genetic screening (PGS)
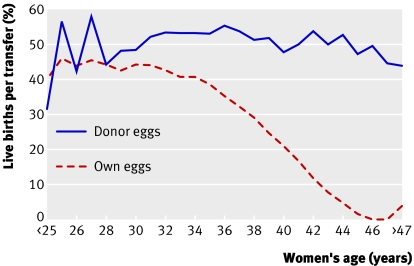
The age related decline in the chances of a live birth after fertility treatment with in vitro fertilisation is related to the decline in the number and quality of eggs. The chances of conception can be restored almost totally by using donor eggs from young women (fig 2). Since sporadic aneuploidy rises with maternal age it was proposed that the use of fluorescence in situ hybridisation to screen embryos for common aneuploidies (chromosomes 13, 16, 18, and 21) in older women would improve the outcome of in vitro fertilisation.13 As a consequence, preimplantation genetic screening for aneuploidy in women who have repeated failure of in vitro fertilisation or repeated miscarriage has become the most common use of embryo biopsy.14
Fig 2 Live birth rates after embryo transfer using own or donor eggs, by age of recipient12
Despite the wide application of screening in patients who are desperate for a successful pregnancy, especially in the United States, until recently it has not been properly validated. Two recent randomised trials found no improvement in the chances of live birth per cycle started; one even showed a reduction.1 15 In part this is explained by the fact that embryo biopsy inevitably reduces the number of embryos available for transfer: some do not survive, test results may be unclear, and mosaicism between embryo cells may result in some normal embryos being identified as unsuitable for transfer.16
Proponents of screening are reluctant to accept these findings, finding fault with the trials' methods and criticising the clinics' ability to perform biopsy. However, a technique that only works in certain enthusiasts' hands and cannot be translated for general use may have little to recommend it.17 Furthermore, those units have not conducted properly controlled studies to show that screening does improve outcome in their hands or which groups of patients benefit.18 Until then, the widespread use of this expensive technology (an additional £2000-£4000; €2880–€5760; $4000-$8000) by in vitro fertilisation centres is arguably unethical.
Inequity of access
Funding for fertility treatment in the UK is in a state of turmoil with the eligibility rules for receiving support varying widely. Unfortunately, preimplantation genetic diagnosis has been caught up in the rationing of funding for fertility treatment, as many primary care trusts do not appreciate the difference between the use of in vitro fertilisation to overcome infertility and its use in fertile couples to avoid the birth of a child with a serious genetic disorder. An innovative arrangement has been achieved in southeast England, where a consortium, informed by a committee of experts, advises on the appropriateness of a request for NHS funding, taking into account the patient's circumstances, the severity of the disorder, and their prospects for success. The consortium generally recommends providing two treatment cycles to couples with a reasonable chance of success—that is, if the woman is younger that 40 at the time of referral and does not already have an unaffected child, which is in line with advice from the Genetics Commissioning Advisory Group.19
Since requests for funding for preimplantation diagnosis are likely to be infrequent, extension of such an arrangement across the UK would help achieve equity and would avoid the understandable confusion with infertility treatment. Better still would be the institution of a national policy and funding through the NHS National Commissioning Group.
Future use
Preimplantation genetic diagnosis is about to change dramatically. Improvements in molecular technology and greater understanding about genetic causes of serious medical disorders will change the referral pattern of couples seeking testing. Until now, it was thought reasonable to limit the use of preimplantation diagnosis to disorders for which prenatal diagnosis was already generally accepted. Future use is likely to expand into areas such as the exclusion of embryos with genes that predispose to adult onset disorders, for which requests for prenatal diagnosis are more unusual. Some people with a genetic predisposition for certain cancers may choose to have preimplantation diagnosis despite the variable penetrance, lethality, and age of onset, and this may even become the largest indication for referral.20
As the success rate improves and the repertoire of diseases for which tests are available increases, the number of couples and their offspring who could benefit from preimplantation diagnosis will rise greatly. It will be especially valuable for couples who would not consider prenatal diagnosis because they find terminating an affected pregnancy unacceptable. However, we will need to ensure that the technique is used only where medically justified.
Summary points
Preimplantation genetic diagnosis can avoid transmission of serious genetic disease
Funding of preimplantation genetic diagnosis needs to be separated from infertility treatment
Demand for testing is likely to grow as technology allows more diseases to be detected
Evidence is lacking that screening for aneuploidy improves the success of in vitro fertilisation in older infertile women
We thank Paul Scriven, Caroline Mackie Ogilvie, Alison Lashwood, and Yakoub Khalaf for helpful comments.
Competing interests: None declared.
Contributors and sources: PB is head of the department of Women's Health at King's College London He is a former member of the Human Fertilisation and Embryology Authority and chair of the scientific advisory committee to the Royal College of Obstetrician and Gynaecologists. FF is a member of the Human Genetics Commission, and has been active in the preimplantation genetic diagnosis centre at Guy's and St Thomas' since its inception.
Provenance and peer review: Commissioned, externally peer reviewed.
This article was posted on bmj.com on 6 September 2007: http://bmj.com/cgi/doi/10.1136/bmj.39314.439491.AD
References
- 1.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med 2007;357:9-17. [DOI] [PubMed] [Google Scholar]
- 2.McLaren A. Prenatal diagnosis before implantation: opportunities and problems. Prenat Diagn 1985;5:85-90. [DOI] [PubMed] [Google Scholar]
- 3.Braude P, Pickering S, Flinter F, Ogilvie CM. Preimplantation genetic diagnosis. Nat Rev Genet 2002;3:941-53. [DOI] [PubMed] [Google Scholar]
- 4.Daniels G, Pettigrew R, Thornhill A, Abbs S, Lashwood A, O'Mahony F, et al. Six unaffected livebirths following preimplantation diagnosis for spinal muscular atrophy. Mol Hum Reprod 2001;7:995-1000. [DOI] [PubMed] [Google Scholar]
- 5.Scriven PN, Handyside AH, Ogilvie CM. Chromosome translocations: segregation modes and strategies for preimplantation genetic diagnosis. Prenat Diagn 1998;18:1437-49. [PubMed] [Google Scholar]
- 6.Handyside AH, Kontogianni EH, Hardy K, Winston RML. Pregnancies from human preimplantation embryos sexed by Y-specific DNA amplification. Nature 1990;344:768-70. [DOI] [PubMed] [Google Scholar]
- 7.Reid T, Landes G, Duckowski W, Klinger K, Ward D. Multicolour fluorescence in situ hybridisation for the simultaneous detection of probe sets for chromosomes 13, 18, 21, X and Y uncultured amniotic fluid cells. Hum Mol Genet 1992;1:307-13. [DOI] [PubMed] [Google Scholar]
- 8.Griffin DK, Handyside AH, Penketh RJA, Winston RML, Delhanty JDA. Fluorescent in-situ hybridization to interphase nuclei of human preimplantation embryos with X and Y chromosome specific probes. Hum Reprod 1991;6:101-5. [DOI] [PubMed] [Google Scholar]
- 9.Renwick P, Ogilvie CM. Preimplantation genetic diagnosis for monogenic diseases: overview and emerging issues. Expert Rev Mol Diagn 2007;7:33-43. [DOI] [PubMed] [Google Scholar]
- 10.Sermon KD, Michiels A, Harton G, Moutou C, Repping S, Scriven PN, et al. ESHRE PGD Consortium data collection VI: cycles from January to December 2003 with pregnancy follow-up to October 2004. Hum Reprod 2007;22:323-36. [DOI] [PubMed] [Google Scholar]
- 11.Grace J, El-Toukhy T, Scriven P, Ogilvie C, Pickering S, Lashwood A, et al. Three hundred and thirty cycles of preimplantation genetic diagnosis for serious genetic disease: clinical considerations affecting outcome. BJOG 2006;113:1393-401. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Assisted reproductive technology (ART) report: section 4—art cycles using donor eggs Atlanta, GA: CDC, 2004. www.cdc.gov/art/ART2004/sect4_fig40-44.htm#f41
- 13.Delhanty JDA, Griffin DK, Handyside AH, Harper J, Atkinson GH, Pieters MH, et al. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation (FISH). Hum Mol Genet 1993;2:1183-5. [DOI] [PubMed] [Google Scholar]
- 14.Kuliev A, Verlinsky Y. Thirteen years' experience of preimplantation diagnosis: report of the fifth international symposium on preimplantation genetics. Reprod Biomed Online 2004;8:229-35. [DOI] [PubMed] [Google Scholar]
- 15.Twisk M, Mastenbroek S, van Wely M, Heineman MJ, Van der Veen F, Repping S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database Syst Rev 2006;(1):CD005291. [DOI] [PubMed]
- 16.Munne S, Velilla E, Colls P, Garcia Bermudez M, Vemuri MC, Steuerwald N, et al. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil Steril 2005;84:1328-34. [DOI] [PubMed] [Google Scholar]
- 17.Thornhill A, Shaw L, Handyside A. PGS: It ain't what you do it's the way that you do it . . . and that's what gets results. BioNews 2007. July 17.
- 18.Donoso P, Staessen C, Fauser BC, Devroey P. Current value of preimplantation genetic aneuploidy screening in IVF. Hum Reprod Update 2007;13:15-25. [DOI] [PubMed] [Google Scholar]
- 19.Department of Health. Preimplantation genetic diagnosis—guiding principles for commissioners of NHS services 2002. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4118934
- 20.Braude P. Preimplantation diagnosis for genetic susceptibility. N Engl J Med 2006;355:541-3. [DOI] [PubMed] [Google Scholar]