Abstract
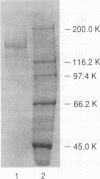
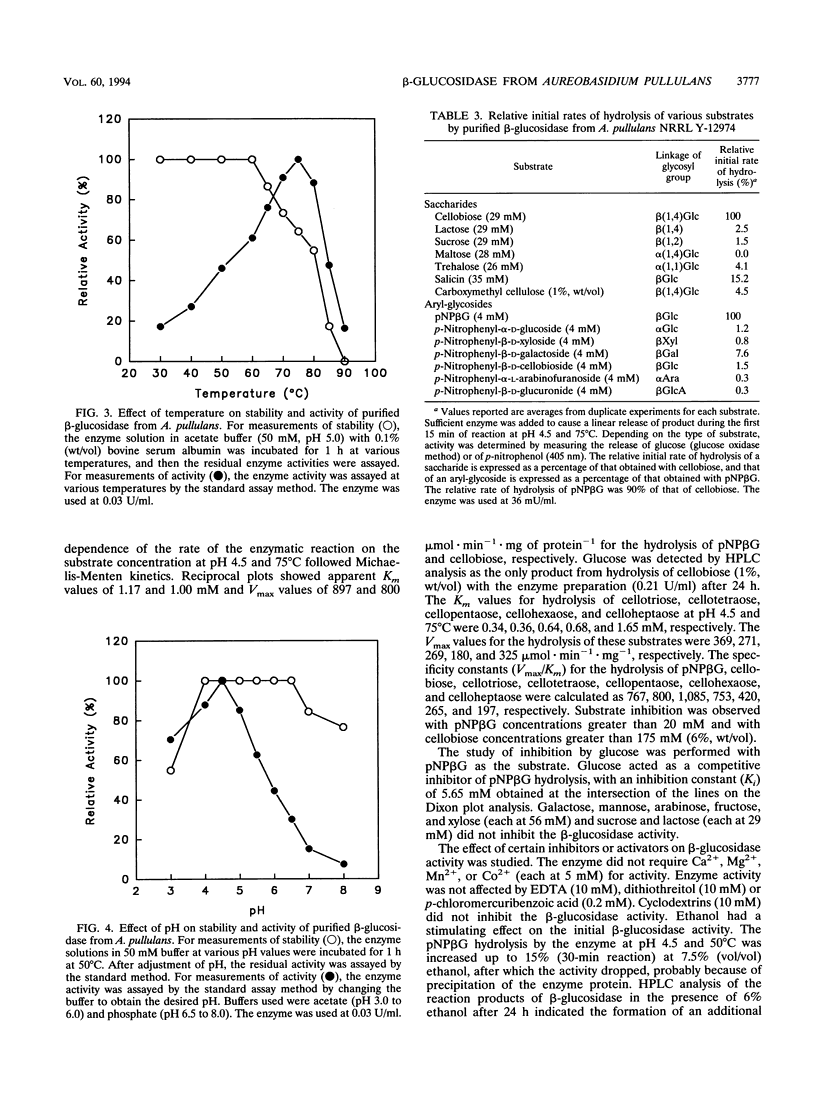
A color variant strain of Aureobasidium pullulans (NRRL Y-12974) produced β-glucosidase activity when grown in liquid culture on a variety of carbon sources, such as cellobiose, xylose, arabinose, lactose, sucrose, maltose, glucose, xylitol, xylan, cellulose, starch, and pullulan. An extracellular β-glucosidase was purified 129-fold to homogeneity from the cell-free culture broth of the organism grown on corn bran. The purification protocol included ammonium sulfate treatment, CM Bio-Gel A agarose column chromatography, and gel filtrations on Bio-Gel A-0.5m and Sephacryl S-200. The β-glucosidase was a glycoprotein with native molecular weight of 340,000 and was composed of two subunits with molecular weights of about 165,000. The enzyme displayed optimal activity at 75°C and pH 4.5 and had a specific activity of 315 μmol · min-1 · mg of protein-1 under these conditions. The purified β-glucosidase was active against p-nitrophenyl-β-d-glucoside, cellobiose, cellotriose, cellotetraose, cellopentaose, cellohexaose, and celloheptaose, with Km values of 1.17, 1.00, 0.34, 0.36, 0.64, 0.68, and 1.65 mM, respectively. The enzyme activity was competitively inhibited by glucose (Ki = 5.65 mM), while fructose, arabinose, galactose, mannose, and xylose (each at 56 mM) and sucrose and lactose (each at 29 mM) were not inhibitory. The enzyme did not require a metal ion for activity, and its activity was not affected by p-chloromercuribenzoate (0.2 mM), EDTA (10 mM), or dithiothreitol (10 mM). Ethanol (7.5%, vol/vol) stimulated the initial enzyme activity by 15%. Glucose production was enhanced by 7.9% when microcrystalline cellulose (2%, wt/vol) was treated for 48 h with a commercial cellulase preparation (5 U/ml) that was supplemented with the purified β-glucosidase (0.21 U/ml) from A. pullulans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A. Amylolytic activity of selected species of ruminal bacteria. Appl Environ Microbiol. 1988 Mar;54(3):772–776. doi: 10.1128/aem.54.3.772-776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drider D., Pommares P., Chemardin P., Arnaud A., Galzy P. Purification and properties of the endocellular beta-glucosidase of Candida cacaoi Buckley and Van Uden CBS 2020. J Appl Bacteriol. 1993 Apr;74(4):473–479. doi: 10.1111/j.1365-2672.1993.tb05156.x. [DOI] [PubMed] [Google Scholar]
- Freer S. N. Purification and characterization of the extracellular beta-glucosidase produced by Candida wickerhamii. Arch Biochem Biophys. 1985 Dec;243(2):515–522. doi: 10.1016/0003-9861(85)90528-4. [DOI] [PubMed] [Google Scholar]
- Kadam S. K., Demain A. L. Addition of cloned beta-glucosidase enhances the degradation of crystalline cellulose by the Clostridium thermocellum cellulose complex. Biochem Biophys Res Commun. 1989 Jun 15;161(2):706–711. doi: 10.1016/0006-291x(89)92657-0. [DOI] [PubMed] [Google Scholar]
- Kengen S. W., Luesink E. J., Stams A. J., Zehnder A. J. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem. 1993 Apr 1;213(1):305–312. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- Kwon K. S., Kang H. G., Hah Y. C. Purification and characterization of two extracellular beta-glucosidases from Aspergillus nidulans. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):149–153. doi: 10.1016/0378-1097(92)90378-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leathers T. D. Color Variants of Aureobasidium pullulans Overproduce Xylanase with Extremely High Specific Activity. Appl Environ Microbiol. 1986 Nov;52(5):1026–1030. doi: 10.1128/aem.52.5.1026-1030.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A. C., Barbier J. R., Willick G. E. Kinetics and specificities of two closely related beta-glucosidases secreted by Schizophyllum commune. Eur J Biochem. 1990 Aug 28;192(1):175–181. doi: 10.1111/j.1432-1033.1990.tb19211.x. [DOI] [PubMed] [Google Scholar]
- Paavilainen S., Hellman J., Korpela T. Purification, characterization, gene cloning, and sequencing of a new beta-glucosidase from Bacillus circulans subsp. alkalophilus. Appl Environ Microbiol. 1993 Mar;59(3):927–932. doi: 10.1128/aem.59.3.927-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painbeni E., Valles S., Polaina J., Flors A. Purification and characterization of a Bacillus polymyxa beta-glucosidase expressed in Escherichia coli. J Bacteriol. 1992 May;174(9):3087–3091. doi: 10.1128/jb.174.9.3087-3091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett M. L., Daniel R. M., Morgan H. W. Purification and properties of a stable beta-glucosidase from an extremely thermophilic anaerobic bacterium. Biochem J. 1987 May 1;243(3):779–787. doi: 10.1042/bj2430779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant A. R., Oliver J. E., Patchett M. L., Daniel R. M., Morgan H. W. Stability and substrate specificity of a beta-glucosidase from the thermophilic bacterium Tp8 cloned into Escherichia coli. Arch Biochem Biophys. 1988 Apr;262(1):181–188. doi: 10.1016/0003-9861(88)90180-4. [DOI] [PubMed] [Google Scholar]
- Rodionova N. A., Tavobilov I. M., Martinovich L. I., Buachidze T. S., Kvesitadze G. I., Bezborodov A. M. beta-Glucosidases from cellulolytic fungi Aspergillus terreus, Geotrichum candidum, and Trichoderma longibrachiatum as typical glycosidases. Biotechnol Appl Biochem. 1987 Jun;9(3):239–250. doi: 10.1111/j.1470-8744.1987.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Ruttersmith L. D., Daniel R. M. Thermostable beta-glucosidase and beta-xylosidase from Thermotoga sp. strain FjSS3-B.1. Biochim Biophys Acta. 1993 Feb 13;1156(2):167–172. doi: 10.1016/0304-4165(93)90132-r. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The octameric structure of beta-glucosidase from Botryodiplodia theobromae Pat. Biochem J. 1991 May 1;275(Pt 3):721–725. doi: 10.1042/bj2750721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Sato T., Yoshioka S., Koshijima T., Kuwahara M. Purification and properties of Aspergillus niger beta-glucosidase. Eur J Biochem. 1992 Oct 15;209(2):651–659. doi: 10.1111/j.1432-1033.1992.tb17332.x. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Yablonsky M. D., Shalita Z. P., Goyal A. K., Eveleigh D. E. Cloning, characterization, and nucleotide sequence of a gene encoding Microbispora bispora BglB, a thermostable beta-glucosidase expressed in Escherichia coli. Appl Environ Microbiol. 1992 Nov;58(11):3455–3465. doi: 10.1128/aem.58.11.3455-3465.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]