Abstract
The ability of iron to catalyze formation of reactive oxygen species significantly contributes to its toxicity in cells and animals. Iron uptake and distribution is regulated tightly in mammalian cells, in part by iron regulatory protein 2 (IRP2), a protein that is degraded efficiently by the proteasome in iron-replete cells. Here, we demonstrate that IRP2 is oxidized and ubiquitinated in cells before degradation. Moreover, iron-dependent oxidation converts IRP2 into a substrate for ubiquitination in vitro. A regulatory pathway is described in which excess iron is sensed by its ability to catalyze site-specific oxidations in IRP2, oxidized IRP2 is ubiquitinated, and ubiquitinated IRP2 subsequently is degraded by the proteasome. Selective targeting and removal of oxidatively modified proteins may contribute to the turnover of many proteins that are degraded by the proteasome.
Many intracellular proteins in eukaryotic cells are degraded by the proteasome (1–5). Degradation rates of individual proteins are often regulated, but the molecular events that determine turnover rates of most of these proteins are ill-defined. Although many proteins destined for proteasomal degradation are multi-ubiquitinated, few specific structural features that predispose to ubiquitination have been identified. Analysis of the degradation of several specific proteins has led to identification of a few distinctive structural features that predispose to ubiquitination, including substrate phosphorylation (6, 7) and changes in the identity of the N-terminal amino acid (8). Thus, analysis of specific protein degradation pathways can facilitate description of distinctive structural features that predispose to recognition by the cellular degradation machinery.
In mammalian cells, iron-regulatory proteins 1 and 2 (IRP1 and 2) sense iron levels and regulate expression of genes of iron metabolism (9, 10). In iron-depleted cells, both IRP1 and IRP2 bind to RNA stem-loops known as iron-responsive elements, inhibiting translation of iron-responsive element-containing transcripts such as ferritin or, alternatively, protecting transferrin receptor mRNA from degradation. However, the mechanism of iron-dependent loss of iron-responsive element-binding activity differs between the two proteins. IRP1 is a stable bifunctional protein in which the presence or absence of an iron–sulfur cluster determines whether IRP1 will function either as the enzyme cytosolic aconitase or as an iron-responsive element-binding protein (9, 10). In contrast, IRP2 is degraded selectively in iron-replete cells and is therefore available to bind transcripts only in iron-depleted cells (11, 12). The sequence responsible for iron-dependent degradation of IRP2, termed the “iron-dependent degradation domain”, consists of a 73-aa sequence unique to IRP2 that is thought to bind iron (13).
To analyze the pathway of iron-dependent degradation of IRP2, we treated cells with proteasome inhibitors and determined that IRP2 protected from degradation is oxidized and ubiquitinated in iron-replete cells. Furthermore, in vitro studies revealed that metal-catalyzed oxidation of IRP2 predisposes to ubiquitination. We outline a pathway for iron-dependent degradation in which efficient iron-dependent oxidation of one or more residues in IRP2 leads to specific ubiquitination of IRP2 and to targeting for proteasomal degradation.
METHODS
Overexpression of IRP1 and IRP2.
Overexpression and purification of IRP1 and IRP2 were done as described (11) with the modification that IRP2 purification was performed in the presence of 100 μM desferrioxamine (Df, an iron-specific chelator) to protect against metal-catalyzed oxidation during the purification process. Oxidation of IRP1 and IRP2 (after dialysis to remove Df) was performed at the concentration of 0.1 μg/μl protein in 20 μl of reaction mixture (25 mM Hepes–NaOH, pH 7.2/40 mM KCl) in the presence of the indicated concentrations of FeCl3 and 10 mM DTT (14). The reaction mixture was incubated at 37°C, and the reaction was stopped by adding Df (100 μM) and cooling to 4°C. Carbonyl groups of oxidized proteins were derivatized with 2,4-dinitrophenylhydrazine (DNP) (Sigma) and were detected by Western blot analysis with anti-DNP antibody (15). A similar experiment was performed in a Coy anaerobic glove box (Coy Laboratory Products, Ann Arbor, MI) and was analyzed for carbonyl formation as described above. In vivo expression of IRP1 and IRP2 constructs was performed as described (13). Transient transfection of COS cells was performed by electroporation. COS cells (5 × 106), suspended in 400 μl of RPMI 1640 medium and 25 mM Hepes (pH 7.4) together with 10 μg of plasmid DNA, were electroporated at 250 V, 500 μF by Gene-Pulser (Bio-Rad) with 0.4-cm electrode gap cuvettes. Df (100 μM) or ferric amminium citrate (FAC) (400 μg/ml) was added to the cultures 24 h after transfection to manipulate cellular iron levels, and cells were harvested 48 hr after transfection.
Identification of Ubiquitinated Proteins.
Extracts for detecting ubiquitinated proteins were prepared by lysing cells with lysing buffer containing 1% Nonidet P-40 (Sigma), 0.5% deoxycholate, 50 mM Tris⋅Cl (pH 8.0), 150 mM NaCl, 0.1% SDS, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 10 mM N-ethylmaleimide on ice for 20 min. Cysteine then was added (final concentration of 0.1%) to neutralize the N-ethylmaleimide. To detect ubiquitinated proteins, protein samples were separated on a 6% SDS/PAGE gel and transferred to poly(vinylidene difluoride) membranes (Bio-Rad). Membranes were fixed with 0.5% glutaraldehyde in 0.1M KPO4 (pH 7.0) at room temperature for 20 min, washed with PBS, blocked with 5% BSA in PBS, and then incubated with rabbit anti-ubiquitin sera, affinity-purified, and diluted as described (16).
The in vitro ubiquitination assay was performed as follows: Native or in vitro-oxidized IRP2 was incubated with 400 μg of RD4 S100 lysates, 5 mM MgCl2, 2 mM ATP, 2 mM DTT, 6 μg of ubiquitin (Sigma), 25 mM Tris⋅Cl (pH 7.6), and 60 mM KCl for 5 min. Reactions were stopped by the addition of 1 ml of ice-cold buffer containing 1% Nonidet P-40, 0.5% deoxycholate, 50 mM Tris⋅Cl (pH 8.0), 150 mM NaCl, and 0.1% SDS. IRP2 was immunoprecipitated with anti-myc antibody and electrophoresed on a 6% SDS/PAGE gel followed by Western blotting with antiubiquitin antibody.
RESULTS
IRP2 Is Subject to Metal-Catalyzed Oxidation in Vitro.
The 73-aa iron-dependent degradation domain of IRP2 contains five cysteines, residues that often are involved in direct ligation of iron. Previous mutagenesis of IRP2 has shown that several of these cysteine residues are required for the process of iron-dependent degradation of IRP2 (13). The presence of a potential iron-binding site in the degradation domain of IRP2 is of interest in considering the mechanism of IRP2 iron-sensing because iron-binding sites in some proteins can promote localized generation of highly reactive hydroxyl radicals by a process referred to as “metal-catalyzed oxidation.” These oxidation reactions share distinctive features: (i) A variety of residues adjacent to the metal binding sites are oxidized; (ii) carbonyl groups are introduced into some of these modified residues, providing a “marker” for oxidative modification; and (iii) oxidative damage often is not inhibited by the addition of free radical scavengers because the reaction is functionally “caged” (17, 18).
Oxidative modification and nonenzymatic cleavages have been described for numerous proteins that contain known metal binding sites (14). In the most extensively studied substrate of metal-catalyzed oxidation reactions, glutamine synthetase of Escherichia coli, modifications include metal-catalyzed oxidation of two histidines to form noncarbonyl-containing products (19) and oxidation of an arginine to yield a carbonyl group (20). In mammalian Cu–Zn superoxide dismutase, a histidine involved in ligation of Cu1+ is sensitive to oxidative modification, leading to damage to the metal-binding site and inactivation of the enzyme (21). Residues that yield carbonyl groups on oxidation, including lysine, arginine, proline, and threonine (18), highly are represented in the 73-aa degradation domain of IRP2, which contains three arginines, five lysines, 10 prolines, and three threonines.
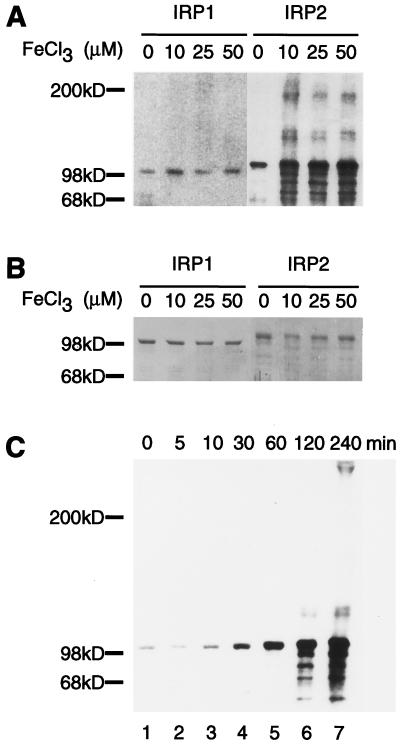
To assess whether a metal-catalyzed oxidation event could play a role in initial sensing of iron levels by IRP2, purified recombinant IRP2 was assayed for formation of carbonyl groups after exposure to iron and reducing agents (15). Consistent with metal-catalyzed oxidation, significant iron-dependent carbonylation of IRP2 but not IRP1 was observed (Fig. 1A). In addition to increases in the carbonyl content of intact IRP2, signals appeared at both higher and lower molecular weights, consistent with oxidative cleavage of the peptide backbone and crosslinking between reactive degradation products of IRP2. Acquisition of carbonyl modifications was not observed when ferric chloride and DTT were added to purified protein under anaerobic conditions, indicating that the process depends on both iron and oxygen (data not shown).
Figure 1.
IRP2 is subject to iron-dependent oxidation in vitro. Purified IRP1 or IRP2 was incubated with 10 mM DTT and FeCl3 at the indicated concentrations for 4 h at 37°C. Oxidative modifications were detected by the carbonyl assay (A), and total protein was detected by Coomassie staining (B). A time course for oxidation of IRP2 shows carbonyl modifications acquired during treatment with 5 μM FeCl3 and 10 mM DTT (C).
To further characterize the dose and time dependence of oxidation, a time course experiment was carried out by using 5 μM ferric chloride, and carbonyl modification was assessed at time points ranging from 5 to 240 min. (Fig. 1C). Carbonyl modifications were detectable at the 5- and 10-min treatment time points in protein that was otherwise intact. As the time of exposure to iron–DTT increased, products derived from cleavage and crosslinking of the peptide backbone appeared. Thus, the initial products of iron-dependent oxidation included full length proteins in which amino acid side chains were oxidized, and longer exposures to iron and reducing agents resulted in more extensive damage to the protein. The efficiency of metal-catalyzed oxidation of IRP2 observed in these experiments supports the inference that an iron binding site is present in IRP2. The stoichiometry of carbonyl modification was determined by using oxidatively modified glutamine synthetase as a standard; carbonyl content of IRP2 at the final time point in the in vitro experiment of Fig. 1C was estimated at ≈1.4 mol of carbonyl group per mol of IRP2 based on comparison to intensity of oxidized glutamine synthetase (15). In contrast, there was no significant increase in iron-dependent oxidative modifications of IRP1 (Fig. 1A).
IRP2 Undergoes Iron-Dependent Oxidation in Vivo.
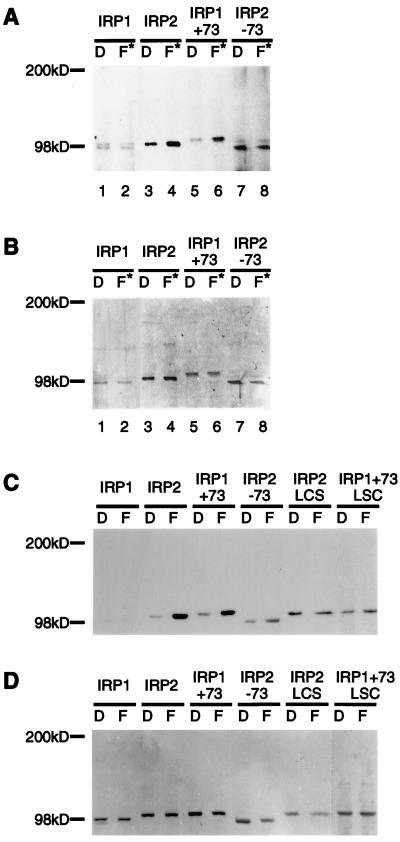
To evaluate whether IRP2 similarly was modified oxidatively on exposure to iron in vivo, IRPs and chimeric constructs that contained or lacked the iron-dependent degradation domain (13) were assessed for iron-dependent acquisition of carbonyl groups in cells treated with either Df or FAC (Fig. 2 A and B). Because previous work has shown that IRP2 is degraded by the proteasome (12, 13), inhibitors of the proteasome were included so that degradation precursors could be characterized. An increase in carbonyl content of IRP2 and IRP1+73 (IRP1 into which the degradation domain has been inserted) was detected in cells treated with iron, but the carbonyl content of IRP1 and IRP2−73 (IRP2 from which the degradation domain is deleted) did not increase with iron treatment (Fig. 2A). IRP2 or IRP1+73 in which three cysteines in the degradation domain are replaced by serines (IRP2–loop cysteines-serines mutations or IRP1+73–loop cysteines-serines mutations) (13) were analyzed for iron-dependent acquisition of carbonyl modifications together with other IRP mutants in transiently transfected COS cells. Of note, only the proteins that contained functional degradation domains acquired carbonyl modifications (Fig. 2 C and D). The carbonyl content of IRP2 and IRP1+73 was estimated as 0.5–1.0 mol of carbonyl per mol of protein based on comparison to glutamine synthetase of known carbonyl content (15), indicating that proteins that contain the degradation domain are oxidized readily in iron-treated cells. Proteins that contained degradation domains in which cysteines were mutated did not acquire carbonyl modifications (Fig. 2C). Thus, only IRP constructs previously identified as substrates for iron-dependent degradation were subject to iron-dependent oxidation, and cysteines of the degradation domain were implicated strongly in facilitating oxidation.
Figure 2.
IRP2 and related proteins are subject to iron-dependent oxidation in vivo. RD4 stable transformants expressing IRP1, IRP2, IRP1+73, and IRP2–73 were cultured for 24 h in the presence of dexamethasone (20 nM) to induce protein expression, then were treated with either Df (D) or FAC plus lactacystin (10 μM) (F*) for 12 h in the continuous presence of dexamethasone. IRPs in cell extracts were quantitated by Western blotting with anti-myc antibody, and equal amounts of recombinant protein from each treatment were immunoprecipitated from the lysates and derivatized by DNP as described in Materials and Methods. Proteins separated on an 8–16% SDS/PAGE were transferred, and carbonyl groups were detected with anti-DNP antibodies (A). Membranes then were stained with Amido Black (Sigma) (B). COS cells transiently transfected with IRP constructs were cultured in the presence of 100 μM Df (D) or 400 μg/ml FAC (F) for 24 h. The amounts of recombinant IRP in cell extracts were determined by Western blot analysis with anti-myc antibody by using purified IRP2 protein as a standard. Immunoprecipitated recombinant proteins (0.5 pmol) were derivatized with DNP, and carbonyl modifications were detected by Western blot analysis with anti-DNP antibody (C). To verify that equivalent amounts of proteins were assayed, membranes then were stained with Coomassie brilliant blue (D).
IRP2 Is Ubiquitinated in Iron-Replete Cells.
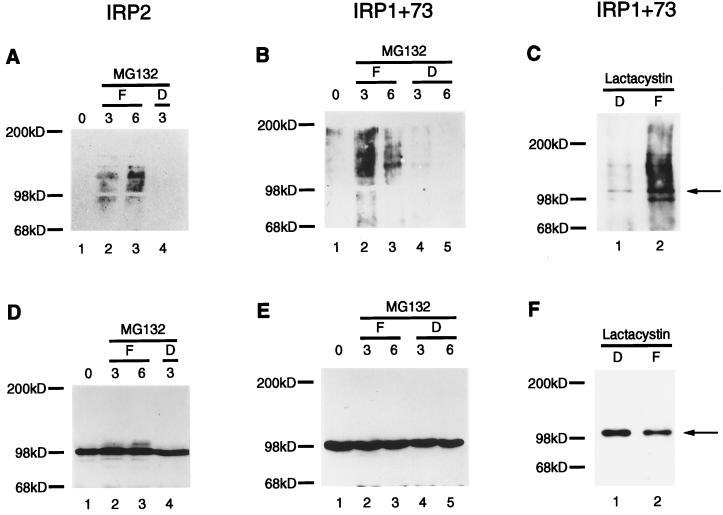
Many proteins that are degraded by the proteasome are subject to ubiquitination (1–5). To determine whether IRP2 was targeted for proteasomal degradation by ubiquitination, cells expressing recombinant IRP2 or other chimeric IRPs (13) were treated simultaneously with the peptide aldehyde proteasomal inhibitor MG132 (23) and with either FAC or Df. Immunoprecipitated IRPs then were assessed for presence of ubiquitin multimers. Notably, multiple higher molecular weight species of IRP2 (Fig. 3A) and IRP1+73 (Fig. 3B) were detected by anti-ubiquitin Western blot analyses (16) of immunoprecipitated IRPs from cells treated with FAC but not Df, indicating that ubiquitination precedes iron-dependent degradation of IRP2 in cells. Similar results were obtained with a structurally unrelated proteasome inhibitor, lactacystin (24) (Fig. 3C). Increased ubiquitination of IRP1 was not observed in cells treated with iron (data not shown), consistent with the observation that IRP1 is not degraded in an iron-dependent fashion.
Figure 3.
IRPs containing the functional degradation domain are ubiquitinated in iron-replete cells. RD4 stable transformants of IRP2 (A and D) or IRP1+73 (B and E) were cultured for 24 hr in the presence of dexamethasone (20 nM) to induce expression of recombinant protein. After induction, MG132 (40 μM) was added for 20 min before the addition of either 100 μg/ml FAC (F, lanes 2 and 3) or 100 μM Df (D, lanes 4 and 5) to individual plates. Lysates of the cells on each plate were prepared at 3 h (A, B, D, and E, lanes 2 and 4) or 6 h (A, B, D, and E, lanes 3 and 5) after adding Df or FAC. Alternatively, after induction of expression with dexamethasone for 24 hr, cells expressing IRP1+73 were treated with lactacystin (5 μM) and either Df (100 μM) or FAC (100 μg/ml) for 12 hr (C and F). Lysates were immunoprecipitated with anti-myc antibody, were resolved by 8% SDS/PAGE, and were transferred and probed with anti-ubiquitin (A–C), followed by stripping and reprobing of the blot with anti-myc antibody (D–F).
Oxidatively Modified IRP2 Is a Substrate for Ubiquitination.
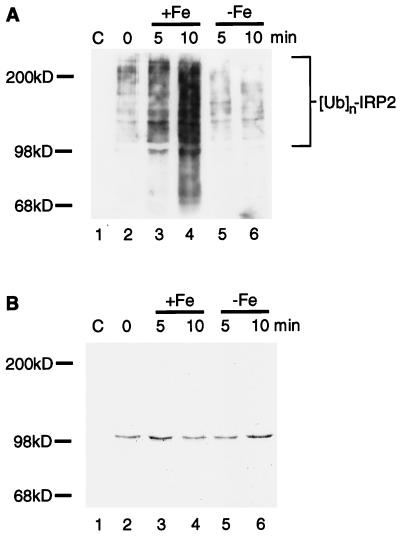
Although IRP2 that was destined to be degraded by the proteasome was observed to be oxidized and ubiquitinated, the in vivo observations did not establish whether oxidation of IRP2 preceded ubiquitination. To address whether oxidative modification(s) of IRP2 renders the protein susceptible to ubiquitination, an in vitro ubiquitination assay was developed in which iron-dependent oxidation of purified IRP2 was performed as a discrete step and iron was chelated before in vitro ubiquitination. Carbonyl modifications of purified IRP2 can be detected after 5–10 min of treatment with FeCl3 and DTT in an otherwise intact protein (Fig. 1C), so IRP2 was treated with FeCl3 and DTT or DTT alone or was left untreated for periods of 5 or 10 min. Subsequently, iron was chelated, and samples were incubated with RD4 cell lysates capable of supporting ubiquitination. IRP2 ubiquitination readily was detected in samples pretreated with iron (Fig. 4A, lanes 3–4) but was only barely detectable in control samples (Fig. 4A, lanes 5 and 6). Coomassie staining of the membrane established that equal amounts of IRP2 were present in all samples (Fig. 4B). The recombinant IRP2 used in the assay was obtained through over expression in baculovirus-infected insect cells and through purification from lysates by column chromatography (11). Desferrioxamine was added to lysis buffer and to buffers used during purification because, in the absence of desferrioxamine, the recombinant IRP2 undergoes progressive degradation similar to the degradation caused by metal-catalyzed oxidation depicted in Fig. 1A. The marked increase in the ubiquitination of the iron-treated protein above the control level suggests that an iron-dependent oxidative modification of IRP2 leads to the creation of recognition signals within IRP2 that are critical for ubiquitination. In addition, these results demonstrate that free iron is not necessary for the subsequent ubiquitination steps once the protein has been oxidized.
Figure 4.
Iron-catalyzed oxidation of IRP2 creates a substrate for in vitro ubiquitination. (A) Purified IRP2 (lanes 2–6) was incubated with 10 mM DTT in the presence or absence of 5 μM FeCl3 at 37°C for 5 min (lanes 3 and 5, respectively) or 10 min (lanes 4 and 6, respectively). Reactions were stopped by addition of 100 μM Df. RD4 cell lysates (400 μg) supplemented with ATP (2 mM) and ubiquitin (6 μg) (Sigma) were added to tubes containing no IRP2 (lane 1), native IRP2 (lane 2), in vitro-oxidized IRP2 (lanes 3 and 4), or IRP2 treated with DTT alone (lanes 5 and 6). Anti-myc immunoprecipitates of each lane were resolved by 6% SDS/PAGE, and Western blotting with antiubiquitin antiserum (A) was followed by staining of the same membrane with Coomassie brilliant blue (B).
DISCUSSION
A number of different strategies are used by cells to regulate levels of iron and copper, in part because regulation of free metal concentrations helps to prevent the catalytic formation of hydroxyl radicals that occurs when these metals react with oxygen species (9). Enzymes important in the detoxification of reactive oxygen species, such as superoxide dismutase and catalase, have been analyzed extensively, and much is known about cellular defenses against oxidation (25).
Substantially less is known about the mechanisms used by cells to dispose of oxidatively damaged components. Recently, the degradation of short-lived proteins in K562 cells was assessed after biosynthetic labeling and exposure to hydrogen peroxide; overall degradation was reported to be increased after hydrogen peroxide treatment, but, when synthesis of 20S proteasomes was inhibited specifically, degradation of proteins decreased, demonstrating that proteasomes are required for degradation of many oxidized proteins (26). However, the molecular basis for targeting of oxidized proteins to the proteasome is not understood. In the case of IRP2, we have demonstrated clearly, by using proteasome inhibitors, that the protein is iron-dependently oxidized and ubiquitinated in cells. Of importance, in vitro studies reveal that oxidation of IRP2 converts IRP2 into a good substrate for ubiquitination. Furthermore, the IRP2 that is oxidized and ubiquitinated in cells is intact, and the cleavage products that were characteristic of extensive iron treatments are not detected under conditions that are sufficient to result in complete iron-dependent degradation of the protein. Thus, IRP2 provides a clear example of an oxidized protein that undergoes ubiquitination in cells before proteasomal degradation.
An important question that will need further evaluation concerns whether the mechanism of IRP2 degradation is unique to IRP2 or whether the steps involved in degradation of IRP2 are generalizable to the full range of cellular proteins that are damaged incidentally as a consequence of iron-dependent oxidation. Our preliminary results suggest that the presence of iron-catalyzed oxidative changes may lead to recognition of many proteins by cellular degradation machinery (data not shown). The rate at which individual proteins are oxidized may be an important determinant of how efficiently the protein is degraded, and the rate of oxidation may depend on whether proteins have surface sites that permit binding of redox active metals. In proteins other than IRP2, infrequent oxidation events could lead to recognition by specific ubiquitin protein ligases similar or identical to those involved in recognition of oxidized IRP2, resulting in culling of randomly oxidized proteins from the cell. The observation that IRP2 is exquisitely sensitive to metal-catalyzed oxidation in vitro correlates with the observed increase in IRP2 oxidation in cells. Ultimately, the ability of IRP2 to function as an iron sensor and regulatory protein depends on an efficient degradation process in which IRP2 is degraded completely by the ubiquitin–proteasome system in iron-replete cells. A similarly efficient oxidation and degradation process also could confer oxygen-sensing potential on hypoxia-inducible factor 1α, an oxygen-sensing transcriptional activator that recently has been shown to be degraded under normoxic and iron-replete conditions by the proteasome (27).
Numerous examples in the literature illustrate the fact that small covalent modifications can alter substantially the functions and fate of proteins. The level of oxidation of IRP2 caused by iron-catalyzed oxidation in vivo is minimal when compared with the effects of high dose in vitro metal-catalyzed oxidation, yet this minimal level of oxidation appears to be sufficient to alter markedly the fate of the protein. Analogous to phosphorylated proteins, such as IκBα, and substrates for the N-end rule, the first step in this process may involve recognition of specific oxidatively modified amino acids by ubiquitin protein ligases. Determining the specific nature of these oxidative modifications and the molecular basis of their recognition by components of the ubiquitin conjugating system should provide insights to how cells respond to oxidative stress. Thus, dissection of the degradation pathway of IRP2 may lead to characterization of ubiquitin enzymes that function at a critical intersection between specific genetic regulatory pathways and general quality control. Detection and specific elimination of proteins that have been damaged oxidatively may represent an important aspect of the cellular defense against oxidation.
Acknowledgments
We thank ProScript (Cambridge, MA) and Dr. Satoshi Omura for MG132 and lactacystin, respectively. We thank Jane Jensen for help with the ubiquitin assay.
ABBREVIATIONS
- IRP
iron regulatory protein
- Df
desferrioxamine
- FAC
ferric amminium citrate
- DNP
2,4-dinitrophenylhydrazine
References
- 1.Rubin D M, Finley D. Curr Biol. 1995;5:854–858. doi: 10.1016/s0960-9822(95)00172-2. [DOI] [PubMed] [Google Scholar]
- 2.Coux O, Tanaka K, Goldberg A L. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 4.Hochstrasser M. Annu Rev Genet. 1996;30:405–440. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 5.Weissman A M. Immunol Today. 1997;18:189–198. doi: 10.1016/s0167-5699(97)84666-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z J, Parent L, Maniatis T. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 8.Madura K, Varshavsky A. Science. 1994;265:1454–1458. doi: 10.1126/science.8073290. [DOI] [PubMed] [Google Scholar]
- 9.Klausner R D, Rouault T A, Harford J B. Cell. 1993;72:19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- 10.Hentze M W, Kuhn L C. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samaniego F, Chin J, Iwai K, Rouault T A, Klausner R D. J Biol Chem. 1994;269:30904–30910. [PubMed] [Google Scholar]
- 12.Guo B, Phillips J D, Yu Y, Leibold E A. J Biol Chem. 1995;270:21645–21651. doi: 10.1074/jbc.270.37.21645. [DOI] [PubMed] [Google Scholar]
- 13.Iwai K, Klausner R D, Rouault T A. EMBO J. 1995;14:5350–5357. doi: 10.1002/j.1460-2075.1995.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K, Rhee S G, Stadtman E R. J Biol Chem. 1985;260:15394–15397. [PubMed] [Google Scholar]
- 15.Levine R L, Williams J A, Stadtman E R, Shacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 16.Cenciarelli C, Wilhelm K G, Jr, Guo A, Weissman A M. J Biol Chem. 1996;271:8709–8713. doi: 10.1074/jbc.271.15.8709. [DOI] [PubMed] [Google Scholar]
- 17.Stadtman E R. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 18.Berlett B S, Stadtman E R. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 19.Rivett A J, Levine R L. Arch Biochem Biophys. 1990;278:26–34. doi: 10.1016/0003-9861(90)90226-o. [DOI] [PubMed] [Google Scholar]
- 20.Climent I, Levine R L. Arch Biochem Biophys. 1991;289:371–5. doi: 10.1016/0003-9861(91)90425-i. [DOI] [PubMed] [Google Scholar]
- 21.Uchida K, Kawakishi S. J Biol Chem. 1994;269:2405–2410. [PubMed] [Google Scholar]
- 22.Breuer W, Epsztejn S, Cabantchik Z I. J Biol Chem. 1995;270:24209–24215. doi: 10.1074/jbc.270.41.24209. [DOI] [PubMed] [Google Scholar]
- 23.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 24.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 25.Scandalios J G, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [Google Scholar]
- 26.Grune T, Reinheckel T, Davies K J A. J Biol Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 27.Salceda S, Caro J. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]