Abstract
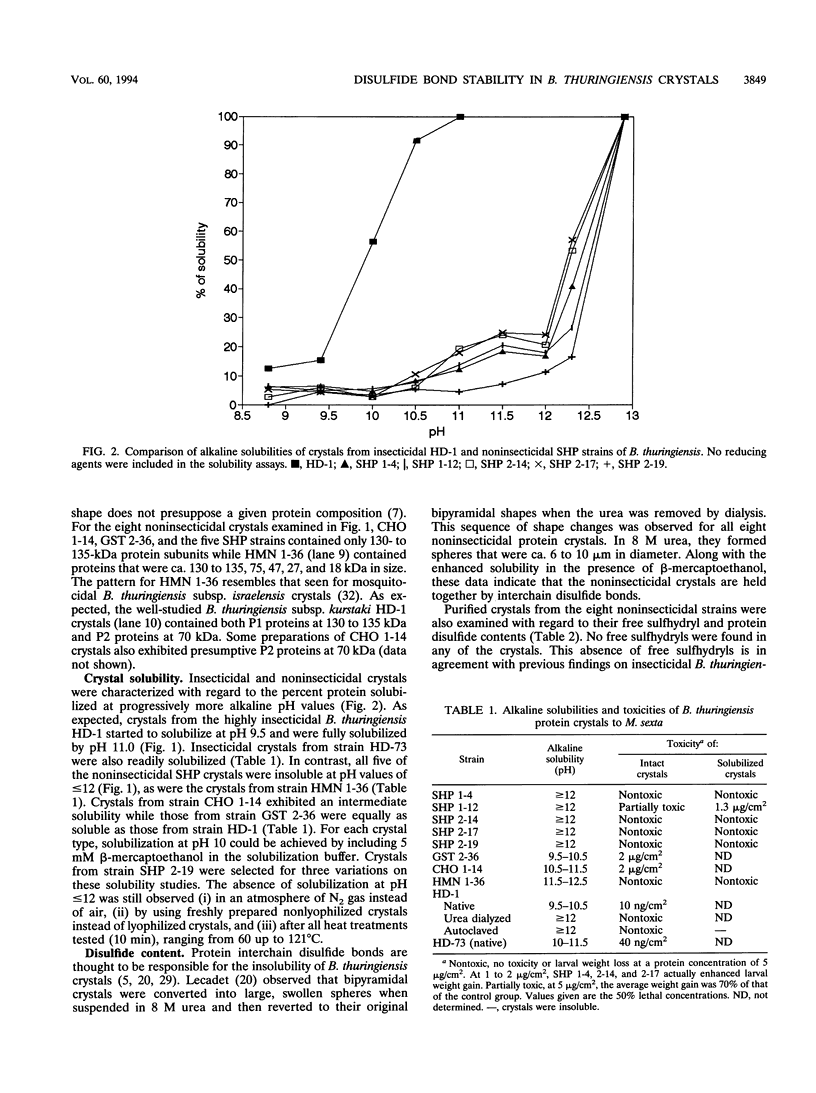
We compared two insecticidal and eight noninsecticidal soil isolates of Bacillus thuringiensis with regard to the solubility of their proteinaceous crystals at alkaline pH values. The protein disulfide contents of the insecticidal and noninsecticidal crystals were equivalent. However, six of the noninsecticidal crystals were soluble only at pH values of ≥12. This lack of solubility contributed to their lack of toxicity. One crystal type which was soluble only at pH ≥12 (strain SHP 1-12) did exhibit significant toxicity to tobacco hornworm larvae when the crystals were presolubilized. In contrast, freshly prepared crystals from the highly insecticidal strain HD-1 were solubilized at pH 9.5 to 10.5, but when these crystals were denatured, by either 8 M urea or autoclave temperatures, they became nontoxic and were soluble only at pH values of ≥12. These changes in toxicity and solubility occurred even though the denatured HD-1 crystals were morphologically indistinguishable from native crystals. Our data are consistent with the view that insecticidal crystals contain distorted, destabilized disulfide bonds which allow them to be solubilized at pH values (9.5 to 10.5) characteristic of lepidopteran and dipteran larval midguts.
Full text
PDF

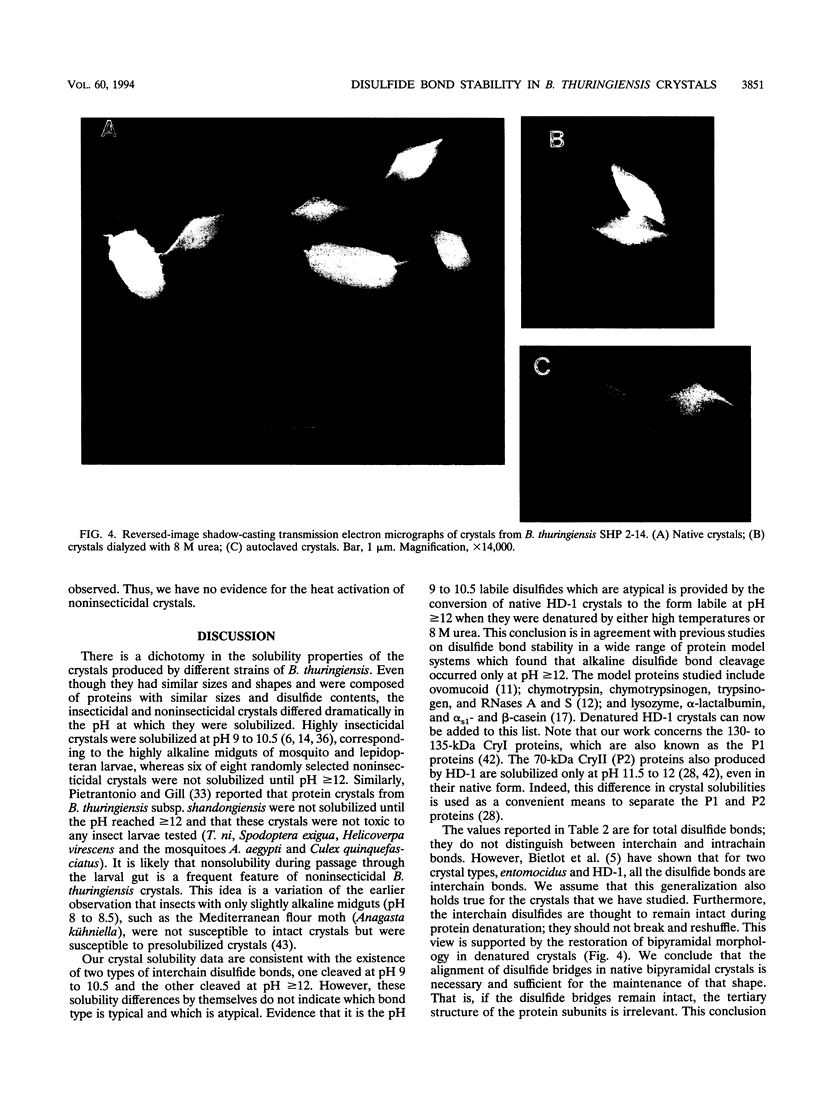


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang B. J., Nickerson K. W. Purification of the protein crystal from Bacillus thuringiensis by zonal gradient centrifugation. Appl Environ Microbiol. 1978 Oct;36(4):625–626. doi: 10.1128/aem.36.4.625-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Han E. S., McGaughey W., Johnson D. The solubility of inclusion proteins from Bacillus thuringiensis is dependent upon protoxin composition and is a factor in toxicity to insects. Appl Environ Microbiol. 1991 Apr;57(4):981–986. doi: 10.1128/aem.57.4.981-986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I. The two faces of Bacillus thuringiensis: insecticidal proteins and post-exponential survival. Mol Microbiol. 1993 Feb;7(4):489–496. doi: 10.1111/j.1365-2958.1993.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Bietlot H. P., Vishnubhatla I., Carey P. R., Pozsgay M., Kaplan H. Characterization of the cysteine residues and disulphide linkages in the protein crystal of Bacillus thuringiensis. Biochem J. 1990 Apr 15;267(2):309–315. doi: 10.1042/bj2670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Kramer K. J., Davidson L. I. Characterization of the entomocidal parasporal crystal of Bacillus thuringiensis. J Bacteriol. 1977 Apr;130(1):375–383. doi: 10.1128/jb.130.1.375-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese D. M., Nickerson K. W., Lane L. C. A comparison of protein crystal subunit sizes in Bacillus thuringiensis. Can J Microbiol. 1980 Aug;26(8):1006–1010. doi: 10.1139/m80-170. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R. Direct analysis of the disulfide content of proteins: methods for monitoring the stability and refolding process of cystine-containing proteins. Anal Biochem. 1991 Aug 15;197(1):52–58. doi: 10.1016/0003-2697(91)90354-v. [DOI] [PubMed] [Google Scholar]
- Crickmore N., Ellar D. J. Involvement of a possible chaperonin in the efficient expression of a cloned CryIIA delta-endotoxin gene in Bacillus thuringiensis. Mol Microbiol. 1992 Jun;6(11):1533–1537. doi: 10.1111/j.1365-2958.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Damodaran S. Estimation of disulfide bonds using 2-nitro-5-thiosulfobenzoic acid: limitations. Anal Biochem. 1985 Feb 15;145(1):200–204. doi: 10.1016/0003-2697(85)90348-3. [DOI] [PubMed] [Google Scholar]
- Dastidar P. G., Nickerson K. W. Interchain crosslinks in the entomocidal Bacillus thuringiensis protein crystal. FEBS Lett. 1979 Dec 15;108(2):411–414. doi: 10.1016/0014-5793(79)80575-x. [DOI] [PubMed] [Google Scholar]
- Donovan J. W., White T. M. Alkaline hydrolysis of the disulfide bonds of ovomucoid and of low molecular weight aliphatic and aromatic disulfides. Biochemistry. 1971 Jan 5;10(1):32–38. doi: 10.1021/bi00777a005. [DOI] [PubMed] [Google Scholar]
- Florence T. M. Degradation of protein disulphide bonds in dilute alkali. Biochem J. 1980 Sep 1;189(3):507–520. doi: 10.1042/bj1890507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Cowles E. A., Pietrantonio P. V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Brown B. J., Carlton B. C. Transfer of Bacillus thuringiensis plasmids coding for delta-endotoxin among strains of B. thuringiensis and B. cereus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6951–6955. doi: 10.1073/pnas.79.22.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lecadet M. M. Action comparée de l'urée et du thioglycolate sur la toxine figurée de Bacillus thuringiensis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jun 12;264(24):2847–2850. [PubMed] [Google Scholar]
- MONRO R. E. Protein turnover and the formation of protein inclusions during sporulation of Bacillus thuringiensis. Biochem J. 1961 Nov;81:225–232. doi: 10.1042/bj0810225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Travers R. S. Worldwide Abundance and Distribution of Bacillus thuringiensis Isolates. Appl Environ Microbiol. 1989 Oct;55(10):2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows M. P., Ellis D. J., Butt J., Jarrett P., Burges H. D. Distribution, Frequency, and Diversity of Bacillus thuringiensis in an Animal Feed Mill. Appl Environ Microbiol. 1992 Apr;58(4):1344–1350. doi: 10.1128/aem.58.4.1344-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls C. N., Ahmad W., Ellar D. J. Evidence for two different types of insecticidal P2 toxins with dual specificity in Bacillus thuringiensis subspecies. J Bacteriol. 1989 Sep;171(9):5141–5147. doi: 10.1128/jb.171.9.5141-5147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W., St Julian G., Bulla L. A., Jr Physiology of sporeforming bacteria associated with insects: radiorespirometric survey of carbohydrate metabolism in the 12 serotypes of Bacillus thuringiensis. Appl Microbiol. 1974 Jul;28(1):129–132. doi: 10.1128/am.28.1.129-132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda K., Inouye K., Ibuchi Y., Oshie K., Shimizu M., Nakamura K., Nishioka R., Takada Y., Ohkawa H. Formation of crystals of the insecticidal proteins of Bacillus thuringiensis subsp. aizawai IPL7 in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3568–3571. doi: 10.1128/jb.171.6.3568-3571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrantonio P. V., Gill S. S. The parasporal inclusion of Bacillus thuringiensis subsp. shandongiensis: characterization and screening for insecticidal activity. J Invertebr Pathol. 1992 May;59(3):295–302. doi: 10.1016/0022-2011(92)90136-r. [DOI] [PubMed] [Google Scholar]
- Priest F. G., Goodfellow M., Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988 Jul;134(7):1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Rogoff M. H., Yousten A. A. Bacillus thuringiensis: microbiological considerations. Annu Rev Microbiol. 1969;23:357–386. doi: 10.1146/annurev.mi.23.100169.002041. [DOI] [PubMed] [Google Scholar]
- Schesser J. H., Kramer K. J., Bulla L. A., Jr Bioassay for homogeneous parasporal crystal of Bacillus thuringiensis using the tobacco hornworm, Manduca sexta. Appl Environ Microbiol. 1977 Apr;33(4):878–880. doi: 10.1128/aem.33.4.878-880.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Couche G. A. The Phylloplane as a Source of Bacillus thuringiensis Variants. Appl Environ Microbiol. 1991 Jan;57(1):311–315. doi: 10.1128/aem.57.1.311-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers R. S., Martin P. A., Reichelderfer C. F. Selective Process for Efficient Isolation of Soil Bacillus spp. Appl Environ Microbiol. 1987 Jun;53(6):1263–1266. doi: 10.1128/aem.53.6.1263-1266.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Federici B. A. A 20-kilodalton protein preserves cell viability and promotes CytA crystal formation during sporulation in Bacillus thuringiensis. J Bacteriol. 1993 Aug;175(16):5276–5280. doi: 10.1128/jb.175.16.5276-5280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Iizuka T. Two types of entomocidal toxins in the parasporal crystals of Bacillus thuringiensis kurstaki. Arch Biochem Biophys. 1983 Nov;227(1):233–241. doi: 10.1016/0003-9861(83)90366-1. [DOI] [PubMed] [Google Scholar]





