Abstract
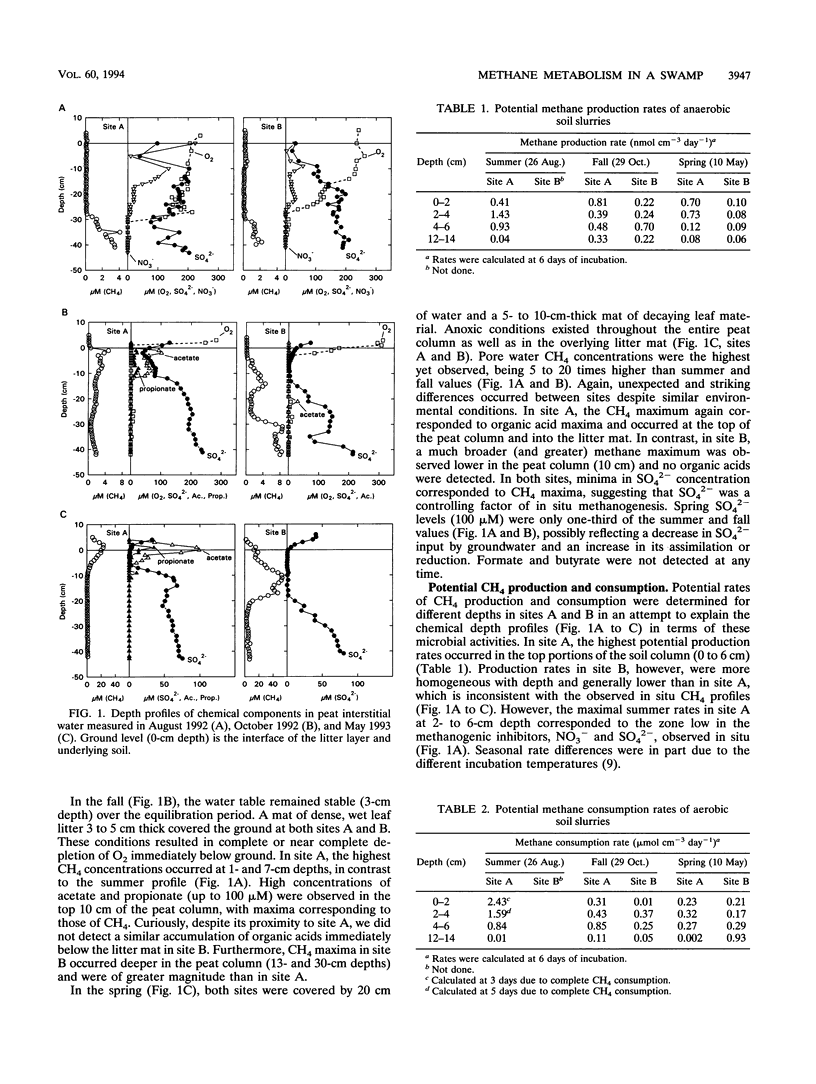
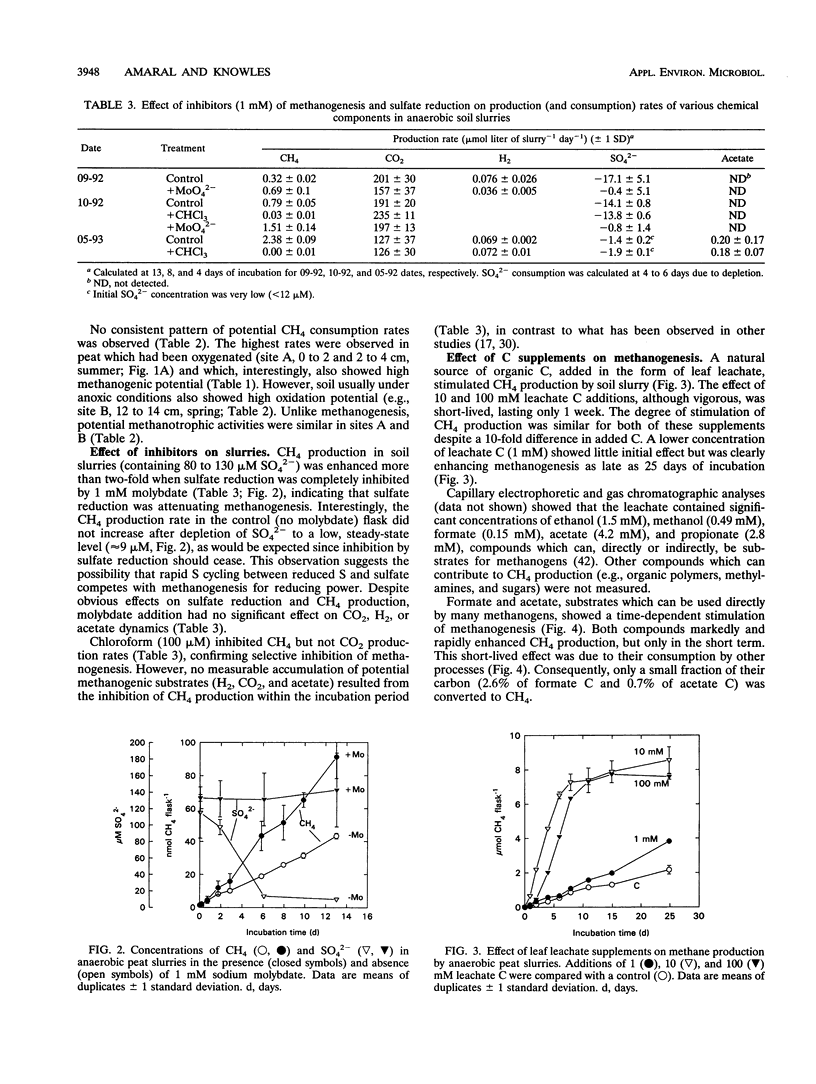
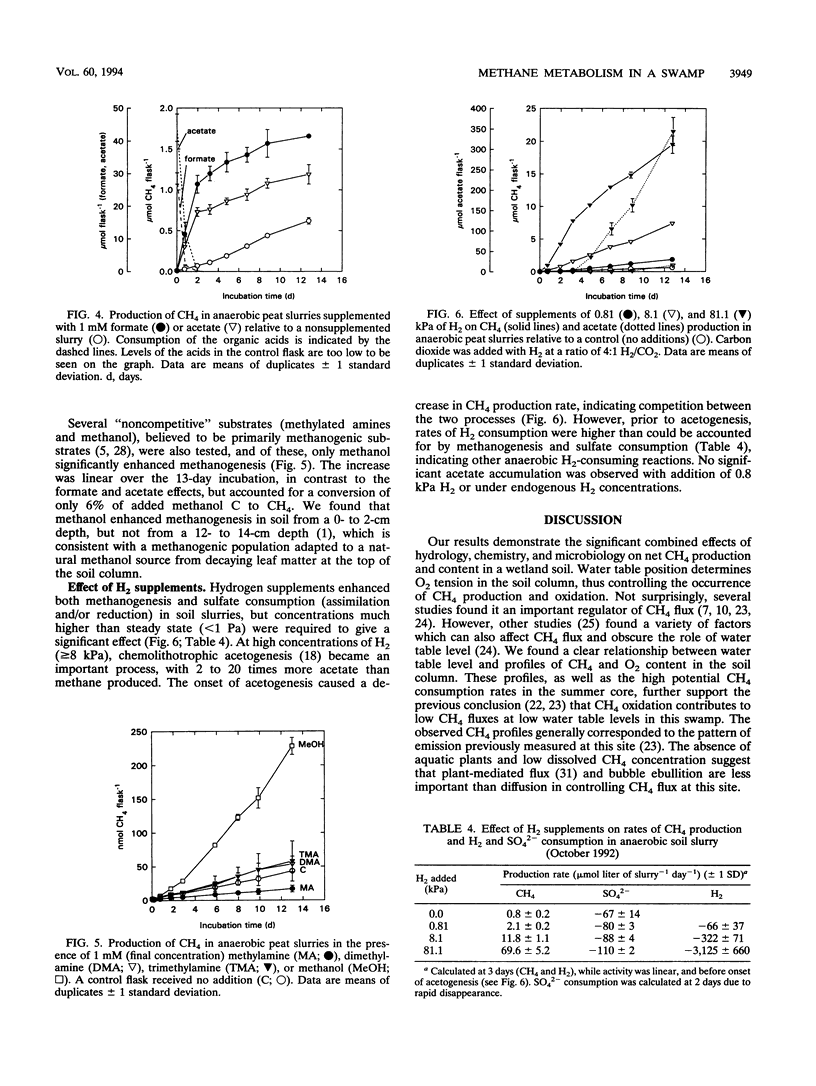
Comparisons between in situ CH4 concentration and potential factors controlling its net production were made in a temperate swamp. Seasonal measurements of water table level and depth profiles of pH, dissolved CH4, CO2, O2, SO42-, NO3-, formate, acetate, propionate, and butyrate were made at two adjacent sites 1.5 to 2 m apart. Dissolved CH4 was inversely correlated to O2 and, in general, to NO3- and SO42-, potential inhibitors of methanogenesis. At low water table levels (August 1992), maximal CH4 (2 to 4 μM) occurred below 30 cm, whereas at high water table levels (October 1992) or under flooded conditions (May 1993), CH4 maxima (4 to 55 μM) occurred in the top 10 to 20 cm. Higher CH4 concentrations were likely supported by inputs of fresh organic matter from decaying leaf litter, as suggested by high acetate and propionate concentrations (25 to 100 μM) in one of the sites in fall and spring. Measurements of potential CH4 production (and consumption) showed that the highest rates generally occurred in the top 10 cm of soil. Soil slurry incubations confirmed the importance of organic matter to CH4 production but also showed that competition for substrates by nonmethanogenic microorganisms could greatly attenuate its effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Payne W. J. Inhibition of methanogenesis in salt marsh sediments and whole-cell suspensions of methanogenic bacteria by nitrogen oxides. Appl Environ Microbiol. 1976 Aug;32(2):264–269. doi: 10.1128/aem.32.2.264-269.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Saulnier M., Ley A. Inhibition of methanogenesis in pure cultures by ammonia, fatty acids, and heavy metals, and protection against heavy metal toxicity by sewage sludge. Can J Microbiol. 1987 Jun;33(6):551–554. doi: 10.1139/m87-093. [DOI] [PubMed] [Google Scholar]
- King G. M., Klug M. J., Lovley D. R. Metabolism of acetate, methanol, and methylated amines in intertidal sediments of lowes cove, maine. Appl Environ Microbiol. 1983 Jun;45(6):1848–1853. doi: 10.1128/aem.45.6.1848-1853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Methanogenesis from methanol and methylamines and acetogenesis from hydrogen and carbon dioxide in the sediments of a eutrophic lake. Appl Environ Microbiol. 1983 Apr;45(4):1310–1315. doi: 10.1128/aem.45.4.1310-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. M., Knowles R. Effect of oxygen and nitrate on nitrogen fixation and denitrification by Azospirillum brasilense grown in continuous culture. Can J Microbiol. 1978 Nov;24(11):1395–1403. doi: 10.1139/m78-223. [DOI] [PubMed] [Google Scholar]
- Westermann P., Ahring B. K. Dynamics of methane production, sulfate reduction, and denitrification in a permanently waterlogged alder swamp. Appl Environ Microbiol. 1987 Oct;53(10):2554–2559. doi: 10.1128/aem.53.10.2554-2559.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. T., Crawford R. L. Methane production in Minnesota peatlands. Appl Environ Microbiol. 1984 Jun;47(6):1266–1271. doi: 10.1128/aem.47.6.1266-1271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



