Abstract
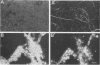
We constructed a small flow chamber in which suboxic medium containing 60 to 120 μM FeCl2 flowed up through a sample well into an aerated reservoir, thereby creating an suboxic-oxic interface similar to the physicochemical conditions that exist in natural iron seeps. When microbial mat material from the Marselisborg iron seep that contained up to 109 bacterial cells per cm3 (D. Emerson and N. P. Revsbech, Appl. Environ. Microbiol. 60:4022-4031, 1994) was placed in the sample well of the chamber, essentially all of the Fe2+ flowing through the sample well was oxidized at rates of up to 1,200 nmol of Fe2+ oxidized per h per cm3 of mat material. The oxidation rates of samples of the mat that were pasteurized prior to inoculation were only about 20 to 50% of the oxidation rates of unpasteurized samples. Sodium azide also significantly inhibited oxidation. These results suggest that at least 50% and up to 80% of the Fe oxidation in the chamber were actively mediated by the microbes in the mat. It also appeared that Fe stimulated the growth of the community since chambers fed with FeCl2 accumulated masses of either filamentous or particulate growth, both in the sample well and attached to the walls of the chamber. Control chambers that did not receive FeCl2 showed no sign of such growth. Furthermore, after 4 to 5 days the chambers fed with FeCl2 contained 35 to 75% more protein than chambers not supplemented with FeCl2. Leptothrix ochracea and, to a lesser extent, Gallionella spp. were responsible for the filamentous growth, and the sheaths and stalks, respectively, of these two organisms harbored large numbers of Fe-encrusted, nonappendaged unicellular bacteria. In chambers where particulate growth predominated, the unicellular bacteria alone appeared to be the primary agents of iron oxidation. These results provide the first clear evidence that the “iron bacteria” commonly found associated with neutral-pH iron seeps are responsible for most of the iron oxidation and that the presence of ferrous iron appears to stimulate the growth of these organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. F., Ghiorse W. C. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J Bacteriol. 1987 Mar;169(3):1279–1285. doi: 10.1128/jb.169.3.1279-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Role of cellular design in bacterial metal accumulation and mineralization. Annu Rev Microbiol. 1989;43:147–171. doi: 10.1146/annurev.mi.43.100189.001051. [DOI] [PubMed] [Google Scholar]
- Boogerd F. C., de Vrind J. P. Manganese oxidation by Leptothrix discophora. J Bacteriol. 1987 Feb;169(2):489–494. doi: 10.1128/jb.169.2.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corstjens P. L., de Vrind J. P., Westbroek P., de Vrind-de Jong E. W. Enzymatic iron oxidation by Leptothrix discophora: identification of an iron-oxidizing protein. Appl Environ Microbiol. 1992 Feb;58(2):450–454. doi: 10.1128/aem.58.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D., Ghiorse W. C. Isolation, Cultural Maintenance, and Taxonomy of a Sheath-Forming Strain of Leptothrix discophora and Characterization of Manganese-Oxidizing Activity Associated with the Sheath. Appl Environ Microbiol. 1992 Dec;58(12):4001–4010. doi: 10.1128/aem.58.12.4001-4010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D., Revsbech N. P. Investigation of an Iron-Oxidizing Microbial Mat Community Located near Aarhus, Denmark: Field Studies. Appl Environ Microbiol. 1994 Nov;60(11):4022–4031. doi: 10.1128/aem.60.11.4022-4031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C. Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol. 1984;38:515–550. doi: 10.1146/annurev.mi.38.100184.002503. [DOI] [PubMed] [Google Scholar]
- Rosson R. A., Tebo B. M., Nealson K. H. Use of poisons in determination of microbial manganese binding rates in seawater. Appl Environ Microbiol. 1984 Apr;47(4):740–745. doi: 10.1128/aem.47.4.740-745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T. Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J Bacteriol. 1968 May;95(5):1921–1942. doi: 10.1128/jb.95.5.1921-1942.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen W. L., Mulder E. G., Deinema M. H. The Sphaerotilus-Leptothrix group of bacteria. Microbiol Rev. 1978 Jun;42(2):329–356. doi: 10.1128/mr.42.2.329-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]