Abstract
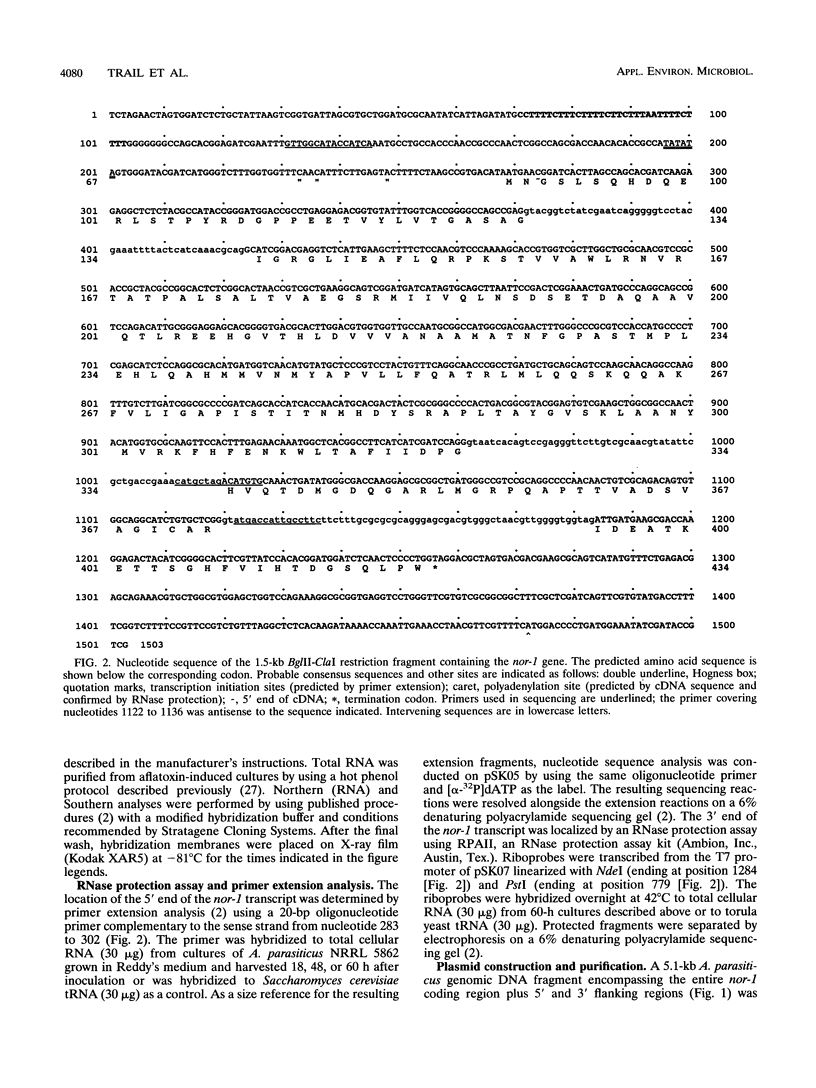
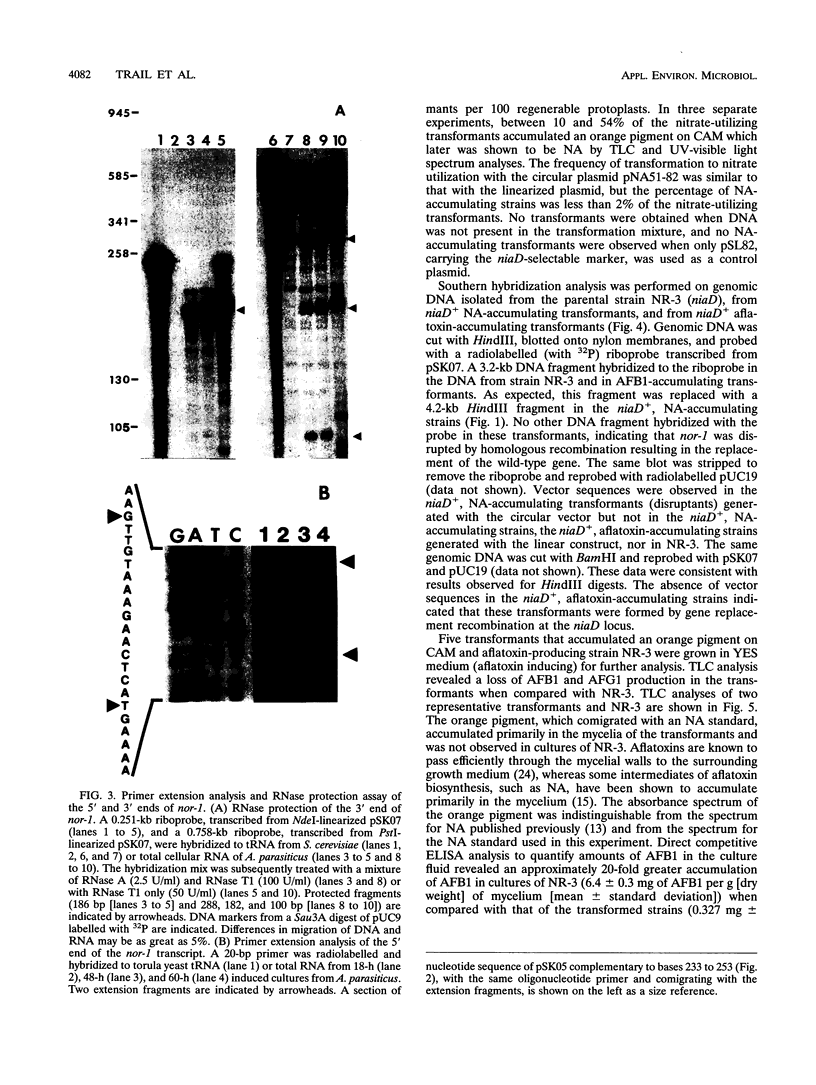
The nor-1 gene was cloned previously by complementation of a mutation (nor-1) in Aspergillus parasiticus SU-1 which blocked aflatoxin B1 biosynthesis, resulting in the accumulation of norsolorinic acid (NA). In this study, the nucleotide sequences of the cDNA and genomic DNA clones encompassing the coding region of the nor-1 gene were determined. The transcription initiation and polyadenylation sites of nor-1 were located by primer extension and RNase protection analyses and by comparison of the nucleotide sequences of the nor-1 genomic and cDNA clones. A plasmid, pNA51-82, was created for one-step disruption of the nor-1 gene by inserting a functional copy of the nitrate reductase (niaD) gene from A. parasiticus into the coding region of the nor-1 gene. Transformation of A. parasiticus NR-3 (niaD Afl+) with pNA51-82 resulted in niaD+ transformants that accumulated NA and produced reduced levels of aflatoxin as determined by thin-layer chromatography and enzyme-linked immunosorbent assay analyses of extracts from mycelia and the growth medium. Southern analysis of genomic DNA isolated from the NA-accumulating transformants indicated that the wild-type nor-1 gene in the chromosome had been replaced by the nonfunctional allele carried on pNA51-82. This recombinational inactivation event provides direct evidence that the nor-1 gene is functionally involved in aflatoxin biosynthesis. Comparison of the predicted nor-1 amino acid sequence with sequences in the GenBank and EMBL databases suggested that the protein is a member of the family of short-chain alcohol dehydrogenases, consistent with its proposed function as a keto reductase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arseculeratne S. N., de Silva L. M., Wijesundera S., Bandunatha C. H. Coconut as a medium for the experimental production of aflatoxin. Appl Microbiol. 1969 Jul;18(1):88–94. doi: 10.1128/am.18.1.88-94.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. L., Jones S. B., Gerasimowicz W. V., Zaika L. L., Stahl H. G., Ocker L. A. Regulation of aflatoxin biosynthesis: assessment of the role of cellular energy status as a regulator of the induction of aflatoxin production. Appl Environ Microbiol. 1987 Jun;53(6):1224–1231. doi: 10.1128/aem.53.6.1224-1231.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. K., Cary J. W., Bhatnagar D., Cleveland T. E., Bennett J. W., Linz J. E., Woloshuk C. P., Payne G. A. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl Environ Microbiol. 1993 Oct;59(10):3273–3279. doi: 10.1128/aem.59.10.3273-3279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. S. Mycotoxins: food contamination, mechanism, carcinogenic potential and preventive measures. Mutat Res. 1991 Mar-Apr;259(3-4):291–306. doi: 10.1016/0165-1218(91)90124-5. [DOI] [PubMed] [Google Scholar]
- Chuturgoon A. A., Dutton M. F., Berry R. K. The preparation of an enzyme associated with aflatoxin biosynthesis by affinity chromatography. Biochem Biophys Res Commun. 1990 Jan 15;166(1):38–42. doi: 10.1016/0006-291x(90)91908-b. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Iyer S. K., Diener U. L. Improved method of screening for aflatoxin with a coconut agar medium. Appl Environ Microbiol. 1987 Jul;53(7):1593–1595. doi: 10.1128/aem.53.7.1593-1595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detroy R. W., Freer S., Ciegler A. Aflatoxin and anthraquinone biosynthesis by nitrosoguanidine-derived mutants of Aspergillus parasiticus. Can J Microbiol. 1973 Nov;19(11):1373–1378. doi: 10.1139/m73-221. [DOI] [PubMed] [Google Scholar]
- Feng G. H., Chu F. S., Leonard T. J. Molecular cloning of genes related to aflatoxin biosynthesis by differential screening. Appl Environ Microbiol. 1992 Feb;58(2):455–460. doi: 10.1128/aem.58.2.455-460.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Horng J. S., Chang P. K., Pestka J. J., Linz J. E. Development of a homologous transformation system for Aspergillus parasiticus with the gene encoding nitrate reductase. Mol Gen Genet. 1990 Nov;224(2):294–296. doi: 10.1007/BF00271564. [DOI] [PubMed] [Google Scholar]
- Keller N. P., Dischinger H. C., Jr, Bhatnagar D., Cleveland T. E., Ullah A. H. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl Environ Microbiol. 1993 Feb;59(2):479–484. doi: 10.1128/aem.59.2.479-484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Bennett J. W., Goldblatt L. A., Lundin R. E. Norsolorinic acid from a mutant strain of Aspergillus parasiticus. J Am Oil Chem Soc. 1971 Feb;48(2):93–94. doi: 10.1007/BF02635696. [DOI] [PubMed] [Google Scholar]
- Lin B. K., Anderson J. A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch Biochem Biophys. 1992 Feb 14;293(1):67–70. doi: 10.1016/0003-9861(92)90366-5. [DOI] [PubMed] [Google Scholar]
- Lloyd A. T., Sharp P. M. Codon usage in Aspergillus nidulans. Mol Gen Genet. 1991 Nov;230(1-2):288–294. doi: 10.1007/BF00290679. [DOI] [PubMed] [Google Scholar]
- Muramatsu M. Preparation of RNA from animal cells. Methods Cell Biol. 1973;7:23–51. doi: 10.1016/s0091-679x(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Rinehart J. E., Mitchell B. L., Oakley C. E., Carmona C., Gray G. L., May G. S. Cloning, mapping and molecular analysis of the pyrG (orotidine-5'-phosphate decarboxylase) gene of Aspergillus nidulans. Gene. 1987;61(3):385–399. doi: 10.1016/0378-1119(87)90201-0. [DOI] [PubMed] [Google Scholar]
- Payne G. A., Nystrom G. J., Bhatnagar D., Cleveland T. E., Woloshuk C. P. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl Environ Microbiol. 1993 Jan;59(1):156–162. doi: 10.1128/aem.59.1.156-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B., Krook M., Jörnvall H. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur J Biochem. 1991 Sep 1;200(2):537–543. doi: 10.1111/j.1432-1033.1991.tb16215.x. [DOI] [PubMed] [Google Scholar]
- Pestka J. J. Enhanced surveillance of foodborne mycotoxins by immunochemical assay. J Assoc Off Anal Chem. 1988 Nov-Dec;71(6):1075–1081. [PubMed] [Google Scholar]
- Reddy T. V., Viswanathan L., Venkitasubramanian T. A. High aflatoxin production on a chemically defined medium. Appl Microbiol. 1971 Sep;22(3):393–396. doi: 10.1128/am.22.3.393-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton N. S., Berry A., Perham R. N. Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. Nature. 1990 Jan 4;343(6253):38–43. doi: 10.1038/343038a0. [DOI] [PubMed] [Google Scholar]
- Sherman D. H., Malpartida F., Bibb M. J., Kieser H. M., Bibb M. J., Hopwood D. A. Structure and deduced function of the granaticin-producing polyketide synthase gene cluster of Streptomyces violaceoruber Tü22. EMBO J. 1989 Sep;8(9):2717–2725. doi: 10.1002/j.1460-2075.1989.tb08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Cary J., Linz J. E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992 Nov;58(11):3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
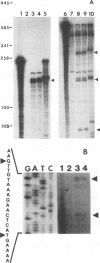
- Skory C. D., Chang P. K., Linz J. E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1993 May;59(5):1642–1646. doi: 10.1128/aem.59.5.1642-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Horng J. S., Pestka J. J., Linz J. E. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl Environ Microbiol. 1990 Nov;56(11):3315–3320. doi: 10.1128/aem.56.11.3315-3320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya A., Juan E., Egestad B., Jörnvall H. The primary structure of alcohol dehydrogenase from Drosophila lebanonensis. Extensive variation within insect 'short-chain' alcohol dehydrogenase lacking zinc. Eur J Biochem. 1989 Mar 1;180(1):191–197. doi: 10.1111/j.1432-1033.1989.tb14632.x. [DOI] [PubMed] [Google Scholar]
- Wu T. S., Linz J. E. Recombinational inactivation of the gene encoding nitrate reductase in Aspergillus parasiticus. Appl Environ Microbiol. 1993 Sep;59(9):2998–3002. doi: 10.1128/aem.59.9.2998-3002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Matsuyama Y., Ando Y., Nakajima H., Hamasaki T. Stereochemistry during aflatoxin biosynthesis: conversion of norsolorinic acid to averufin. Appl Environ Microbiol. 1993 Aug;59(8):2486–2492. doi: 10.1128/aem.59.8.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Otake H., Koyama Y., Horiuchi T., Nakano E. Cloning, sequencing, and expression of the N-acyl-D-mannosamine dehydrogenase gene from Flavobacterium sp. strain 141-8 in Escherichia coli. Appl Environ Microbiol. 1991 May;57(5):1418–1422. doi: 10.1128/aem.57.5.1418-1422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Cary J. W., Bhatnagar D., Cleveland T. E., Keller N. P., Chu F. S. Cloning and characterization of a cDNA from Aspergillus parasiticus encoding an O-methyltransferase involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1993 Nov;59(11):3564–3571. doi: 10.1128/aem.59.11.3564-3571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]