Abstract
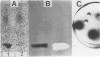
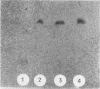
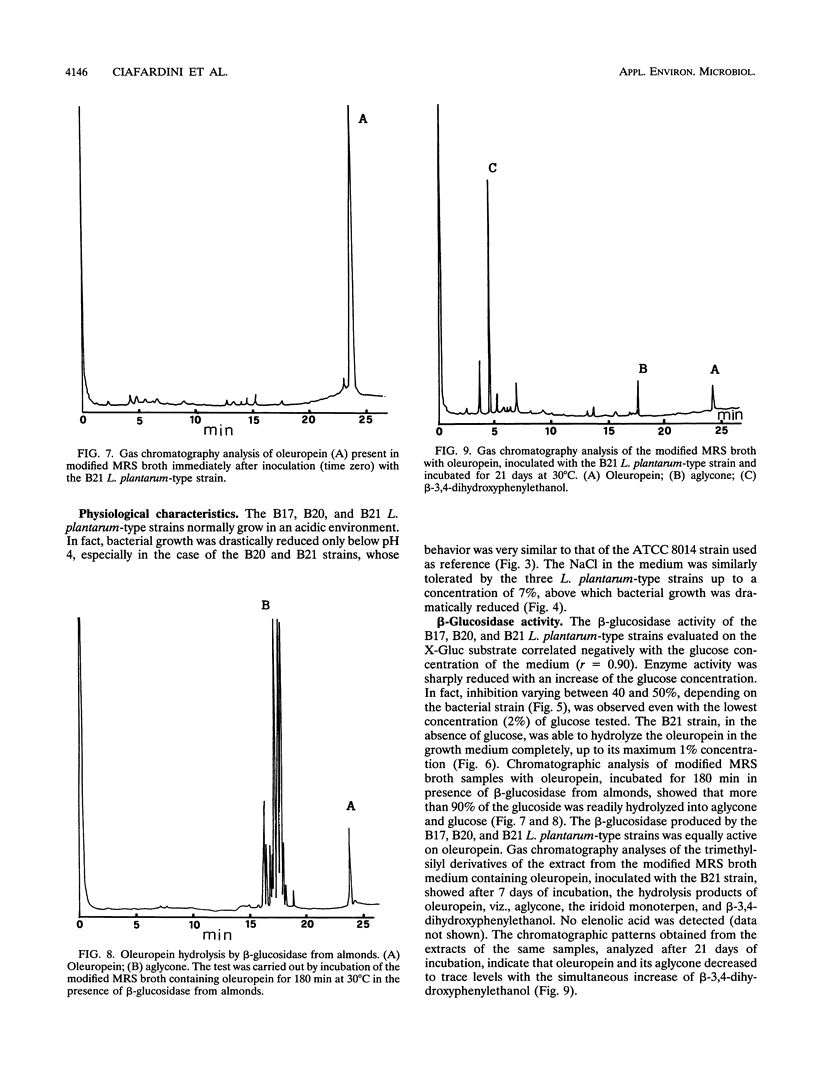
Oleuropein (Chemical Abstracts Service registry number 32619-42-4), a bitter-tasting secoiridoid glucoside commonly found in leaves of the olive tree as well as in olives (Olea europaea L.), was found to be hydrolyzed by the β-glucosidase (EC 3.2.1.2.1) produced by oleuropeinolytic Lactobacillus plantarum-type strains. Three strains, designated B17, B20, and B21, were isolated from the brine of naturally ripe olives not treated with alkali. These strains were rod-shaped forms, grown at a pH 3.5 limit, and tolerated 1% oleuropein and 8% NaCl in the growth medium. The β-glucosidase produced hydrolyzed 5-bromo-4-chloro-3-indolyl-β-d-glucopy-ranoside as well as oleuropein. The presence of 2% glucose in the medium inhibited activity by 40 to 50%, depending on the bacterial strain. Chromatographic analysis of the trimethylsilyl derivatives of the products obtained after 7 days of incubation at 30°C of strain B21 showed all the hydrolysis products of oleuropein, i.e., aglycone, iridoid monoterpen, and 3,4-dihydroxyphenylethanol (hydroxytyrosol). Oleuropein and its aglycone after 21 days of incubation decreased to trace levels with the simultaneous increase in concentration of β-3,4-dihydroxyphenylethanol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLECHER M., GLASSMAN A. B. Determination of glucose in the presence of sucrose using glucose oxidase; effect of pH on absorption spectrum of oxidized o-dianisidine. Anal Biochem. 1962 Apr;3:343–352. doi: 10.1016/0003-2697(62)90119-7. [DOI] [PubMed] [Google Scholar]
- Etchells J. L., Borg A. F., Kittel I. D., Bell T. A., Fleming H. P. Pure culture fermentation of green olives. Appl Microbiol. 1966 Nov;14(6):1027–1041. doi: 10.1128/am.14.6.1027-1041.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici F., Bongi G. Improved method for isolation of bacterial inhibitors from oleuropein hydrolysis. Appl Environ Microbiol. 1983 Aug;46(2):509–510. doi: 10.1128/aem.46.2.509-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Gonzalez M. J., García García P., Garrido Fernández A., Durán Quintana M. C. Microflora of the aerobic preservation of directly brined green olives from Hojiblanca cultivar. J Appl Bacteriol. 1993 Sep;75(3):226–233. doi: 10.1111/j.1365-2672.1993.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Fleming H. P., Etchells J. L. Occurrence of an inhibitor of lactic Acid bacteria in green olives. Appl Microbiol. 1967 Sep;15(5):1178–1184. doi: 10.1128/am.15.5.1178-1184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H. P., Walter W. M., Jr, Etchells J. L. Antimicrobial properties of oleuropein and products of its hydrolysis from green olives. Appl Microbiol. 1973 Nov;26(5):777–782. doi: 10.1128/am.26.5.777-782.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Fernandez A., Vaughn R. H. Utilization of oleuropein by microorganisms associated with olive fermentations. Can J Microbiol. 1978 Jun;24(6):680–684. doi: 10.1139/m78-114. [DOI] [PubMed] [Google Scholar]
- Juven B., Henis Y., Jacoby B. Studies on the mechanism of the antimicrobial action of oleuropein. J Appl Bacteriol. 1972 Dec;35(4):559–567. doi: 10.1111/j.1365-2672.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- McDonald L. C., Fleming H. P., Hassan H. M. Acid Tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol. 1990 Jul;56(7):2120–2124. doi: 10.1128/aem.56.7.2120-2124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A., WISEMAN R. F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951 Jul;62(1):132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter W. M., Jr, Fleming H. P., Etchells J. L. Preparation of antimicrobial compounds by hydrolysis of oleuropein from green olives. Appl Microbiol. 1973 Nov;26(5):773–776. doi: 10.1128/am.26.5.773-776.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y Alcaĺa J. M., Ferńandez-Diez M. J., Gonźalez-Cancho F. Influence of pasteurization and lye treatment on the fermentation of spanish-style manzanilla olives. Appl Microbiol. 1969 May;17(5):734–736. doi: 10.1128/am.17.5.734-736.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]