Abstract
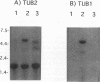
Colletotrichum gloeosporioides f. sp. aeschynomene is a fungal plant pathogen of Aeschynomene virginica. A beta-tubulin-encoding gene (TUB2) from this pathogen was cloned and sequenced. The deduced amino acid sequence of TUB2 had a high degree of homology to other fungal beta-tubulins. A portion of TUB2 from a benomyl-resistant C. gloeosporioides f. sp. aeschynomene mutant was also cloned and sequenced. A point mutation resulting in a glutamic acid-to-lysine substitution at amino acid 198 likely confers benomyl resistance. The mutation is relevant for use as a selectable marker in developing a gene transfer system in C. gloeosporioides f. sp. aeschynomene. Northern (RNA) hybridizations with C. gloeosporioides f. sp. aeschynomene TUB2 and another C. gloeosporioides f. sp. aeschynomene beta-tubulin-encoding gene (TUB1) as probes showed differential expression of these genes in different cell types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballance D. J. Sequences important for gene expression in filamentous fungi. Yeast. 1986 Dec;2(4):229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- Buhr T. L., Dickman M. B. Isolation and characterization of a beta-tubulin-encoding gene from Colletotrichum gloeosporioides f. sp. aeschynomene. Gene. 1993 Feb 14;124(1):121–125. doi: 10.1016/0378-1119(93)90771-t. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fujimura M., Oeda K., Inoue H., Kato T. A single amino-acid substitution in the beta-tubulin gene of Neurospora confers both carbendazim resistance and diethofencarb sensitivity. Curr Genet. 1992 Apr;21(4-5):399–404. doi: 10.1007/BF00351701. [DOI] [PubMed] [Google Scholar]
- Jung M. K., Wilder I. B., Oakley B. R. Amino acid alterations in the benA (beta-tubulin) gene of Aspergillus nidulans that confer benomyl resistance. Cell Motil Cytoskeleton. 1992;22(3):170–174. doi: 10.1002/cm.970220304. [DOI] [PubMed] [Google Scholar]
- May G. S., Gambino J., Weatherbee J. A., Morris N. R. Identification and functional analysis of beta-tubulin genes by site specific integrative transformation in Aspergillus nidulans. J Cell Biol. 1985 Sep;101(3):712–719. doi: 10.1083/jcb.101.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Polycistronic transcripts in trypanosomes and their accumulation during heat shock: evidence for a precursor role in mRNA synthesis. Mol Cell Biol. 1988 Sep;8(9):3837–3846. doi: 10.1128/mcb.8.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Van Etten J. L., Grabherr R. DNA sequencing of four bases using three lanes. Nucleic Acids Res. 1992 Mar 25;20(6):1345–1348. doi: 10.1093/nar/20.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaccione D. G., Hanau R. M. Characterization of two divergent beta-tubulin genes from Colletotrichum graminicola. Gene. 1990 Feb 14;86(2):163–170. doi: 10.1016/0378-1119(90)90275-v. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden O., Plamann M., Ebbole D. J., Yanofsky C. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 1992 Jun;11(6):2159–2166. doi: 10.1002/j.1460-2075.1992.tb05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]