Abstract
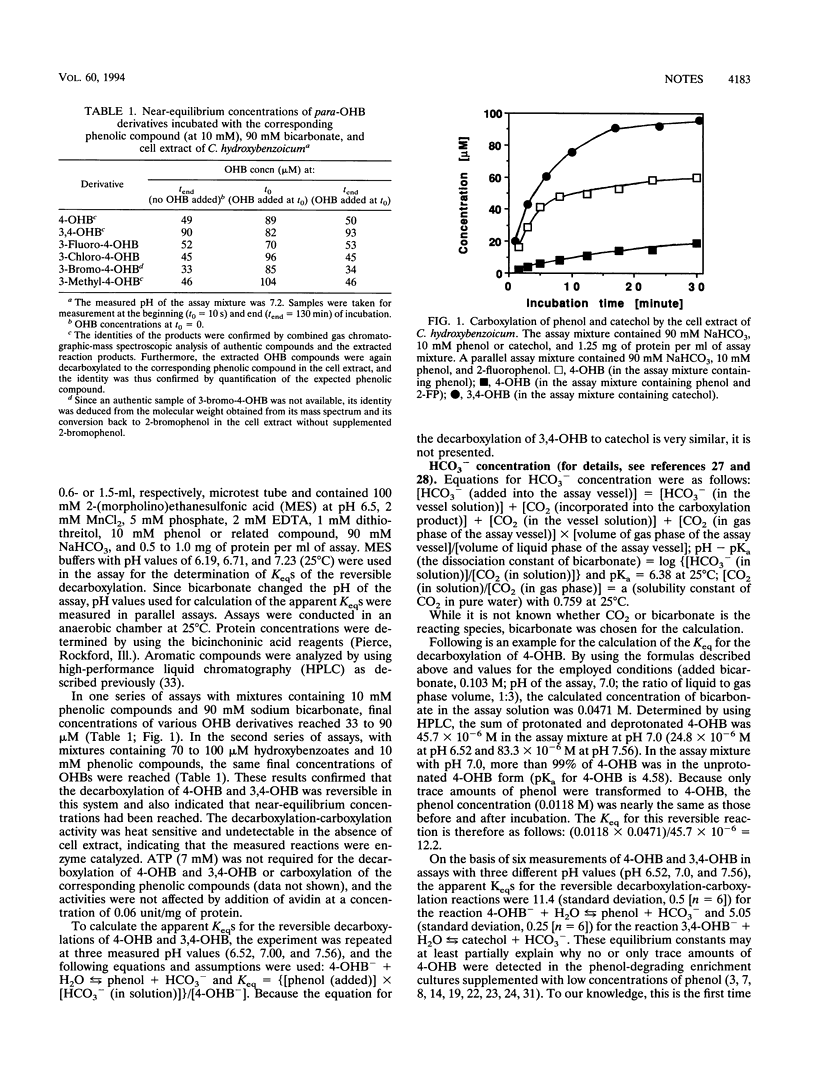
Reversible conversion of 4-hydroxybenzoate and phenol and their analogs was observed in whole-cell suspensions and cell extracts of Clostridium hydroxybenzoicum grown with 4-hydroxybenzoate and 3,4-dihydroxybenzoate. Assuming that bicarbonate is the cosubstrate, the equilibrium constants calculated for the reactions 4-hydroxybenzoate- + H2O ⇆ phenol + HCO3- and 3,4-dihydroxybenzoate- + H2O ⇆ catechol + HCO3- were 11.4 (± 0.5) and 5.05 (± 0.25), respectively. In a phenol-adapted sediment slurry, 4-hydroxybenzoate and 3,4-dihydroxybenzoate were decarboxylated to phenol and to catechol, respectively, as intermediates without a lag time.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. The methanogenic biodegradation of catechol by a microbial consortium: evidence for the production of phenol through cis-benzenediol. Biochem Soc Trans. 1980 Aug;8(4):452–453. doi: 10.1042/bst0080452. [DOI] [PubMed] [Google Scholar]
- Bisaillon J. G., Lépine F., Beaudet R., Sylvestre M. Carboxylation of o-cresol by an anaerobic consortium under methanogenic conditions. Appl Environ Microbiol. 1991 Aug;57(8):2131–2134. doi: 10.1128/aem.57.8.2131-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaillon J. G., Lépine F., Beaudet R., Sylvestre M. Potential for carboxylation-dehydroxylation of phenolic compounds by a methanogenic consortium. Can J Microbiol. 1993 Jul;39(7):642–648. doi: 10.1139/m93-093. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Anaerobic Degradation of Chloroaromatic Compounds in Aquatic Sediments under a Variety of Enrichment Conditions. Appl Environ Microbiol. 1989 Jun;55(6):1466–1471. doi: 10.1128/aem.55.6.1466-1471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Townsend G. T., Chapman P. J. Anaerobic transformation of phenol to benzoate via para-carboxylation: use of fluorinated analogues to elucidate the mechanism of transformation. Biochem Biophys Res Commun. 1989 Aug 15;162(3):945–951. doi: 10.1016/0006-291x(89)90764-x. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Townsend G. T., Chapman P. J. para-hydroxybenzoate as an intermediate in the anaerobic transformation of phenol to benzoate. FEMS Microbiol Lett. 1991 Mar 1;62(2-3):265–269. doi: 10.1016/0378-1097(91)90168-a. [DOI] [PubMed] [Google Scholar]
- Glöckler R., Tschech A., Fuchs G. Reductive dehydroxylation of 4-hydroxybenzoyl-CoA to benzoyl-CoA in a denitrifying, phenol-degrading Pseudomonas species. FEBS Lett. 1989 Jul 17;251(1-2):237–240. doi: 10.1016/0014-5793(89)81461-9. [DOI] [PubMed] [Google Scholar]
- Grant D. J., Patel J. C. The non-oxidative decarboxylation of p-hydroxybenzoic acid, gentisic acid, protocatechuic acid and gallic acid by Klebsiella aerogenes (Aerobacter aerogenes). Antonie Van Leeuwenhoek. 1969;35(3):325–343. doi: 10.1007/BF02219153. [DOI] [PubMed] [Google Scholar]
- Hsu T. D., Lux M. F., Drake H. L. Expression of an aromatic-dependent decarboxylase which provides growth-essential CO2 equivalents for the acetogenic (Wood) pathway of Clostridium thermoaceticum. J Bacteriol. 1990 Oct;172(10):5901–5907. doi: 10.1128/jb.172.10.5901-5907.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T., Daniel S. L., Lux M. F., Drake H. L. Biotransformations of carboxylated aromatic compounds by the acetogen Clostridium thermoaceticum: generation of growth-supportive CO2 equivalents under CO2-limited conditions. J Bacteriol. 1990 Jan;172(1):212–217. doi: 10.1128/jb.172.1.212-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack A., Fuchs G. Carboxylation of phenylphosphate by phenol carboxylase, an enzyme system of anaerobic phenol metabolism. J Bacteriol. 1992 Jun;174(11):3629–3636. doi: 10.1128/jb.174.11.3629-3636.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack A., Tommasi I., Aresta M., Fuchs G. Catalytic properties of phenol carboxylase. In vitro study of CO2: 4-hydroxybenzoate isotope exchange reaction. Eur J Biochem. 1991 Apr 23;197(2):473–479. doi: 10.1111/j.1432-1033.1991.tb15934.x. [DOI] [PubMed] [Google Scholar]
- Londry K. L., Fedorak P. M. Benzoic acid intermediates in the anaerobic biodegradation of phenols. Can J Microbiol. 1992 Jan;38(1):1–11. doi: 10.1139/m92-001. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Lonergan D. J. Anaerobic Oxidation of Toluene, Phenol, and p-Cresol by the Dissimilatory Iron-Reducing Organism, GS-15. Appl Environ Microbiol. 1990 Jun;56(6):1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharak Genthner B. R., Townsend G. T., Chapman P. J. Effect of fluorinated analogues of phenol and hydroxybenzoates on the anaerobic transformation of phenol to benzoate. Biodegradation. 1990;1(1):65–74. doi: 10.1007/BF00117052. [DOI] [PubMed] [Google Scholar]
- Tschech A., Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987 Sep;148(3):213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Saiki T., Liu S. M., Ljungdahl L. G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983 Feb 10;258(3):1826–1832. [PubMed] [Google Scholar]
- Zhang X., Mandelco L., Wiegel J. Clostridium hydroxybenzoicum sp. nov., an amino acid-utilizing, hydroxybenzoate-decarboxylating bacterium isolated from methanogenic freshwater pond sediment. Int J Syst Bacteriol. 1994 Apr;44(2):214–222. doi: 10.1099/00207713-44-2-214. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wiegel J. Sequential anaerobic degradation of 2,4-dichlorophenol in freshwater sediments. Appl Environ Microbiol. 1990 Apr;56(4):1119–1127. doi: 10.1128/aem.56.4.1119-1127.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



