Abstract
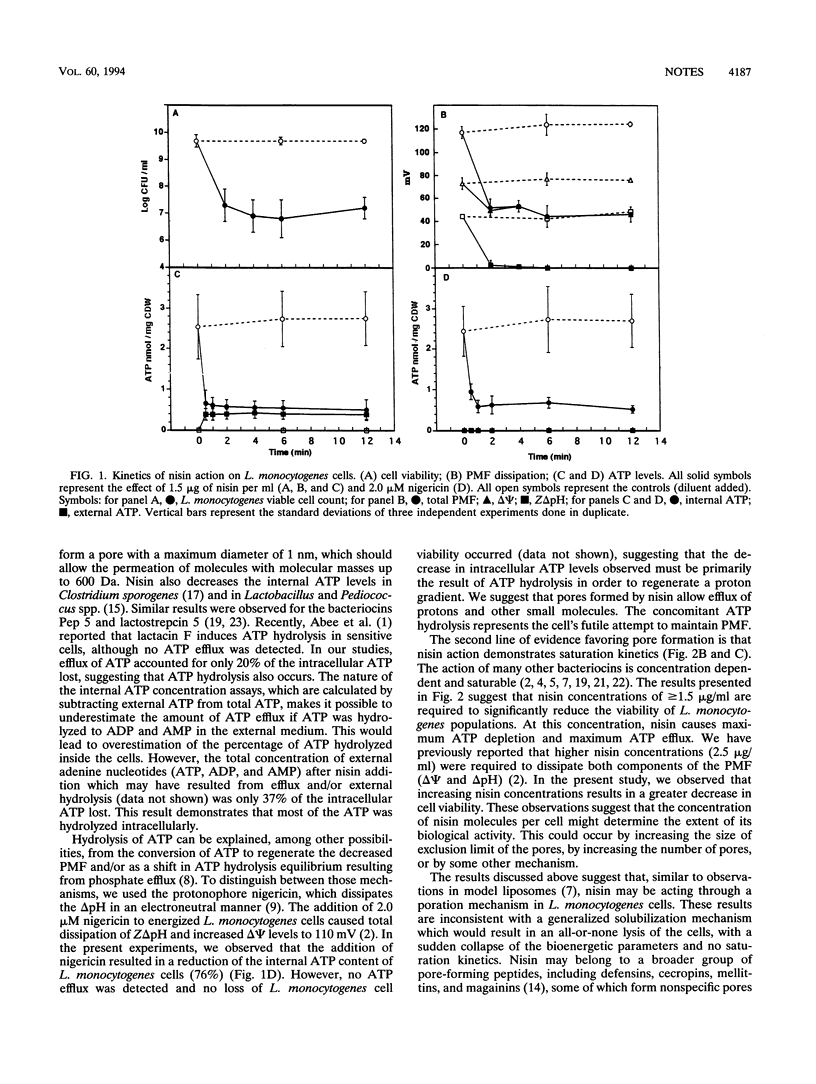
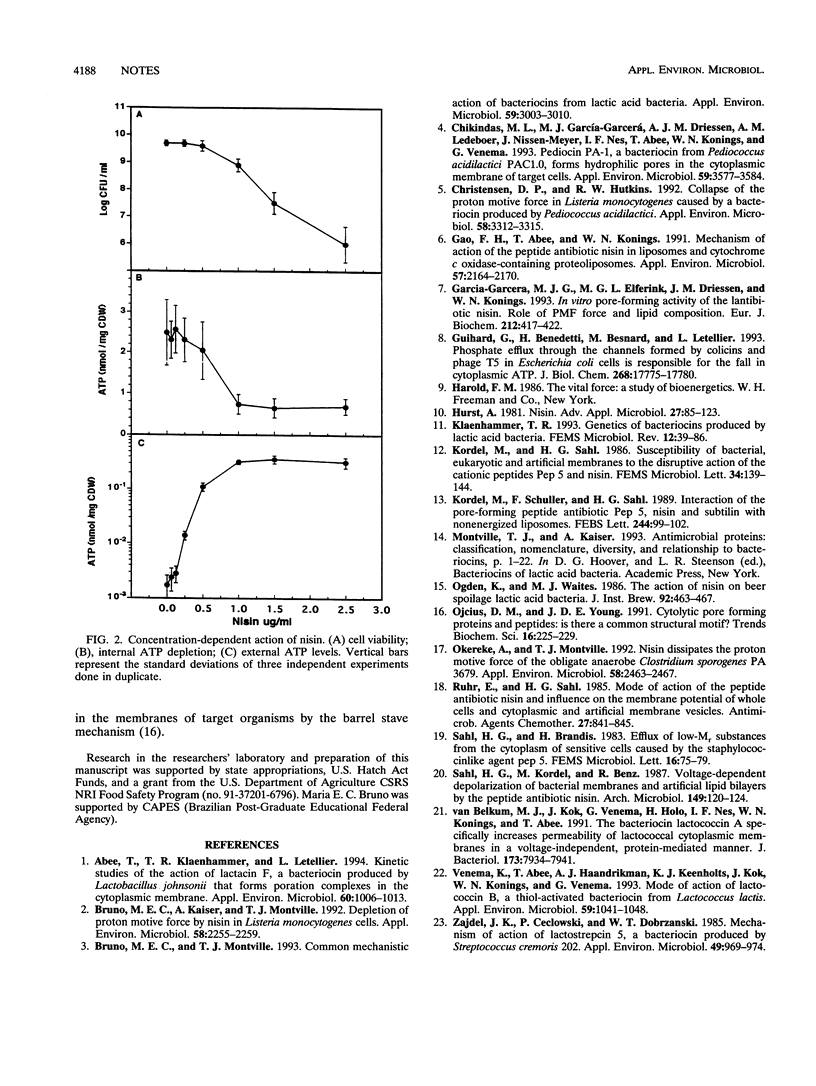
In Listeria monocytogenes, nisin induced ATP efflux, reduced the intracellular ATP concentration within 1 min, and dissipated the proton motive force within 2 min. Efflux accounted for only 20% of the ATP depletion, suggesting that ATP hydrolysis also occurred. ATP efflux depended on nisin concentration and followed saturation kinetics. These results suggest that nisin breaches the membrane permeability barrier in a manner more consistent with pore formation than with a nonspecific detergent-like membrane destabilization.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abee T., Klaenhammer T. R., Letellier L. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl Environ Microbiol. 1994 Mar;60(3):1006–1013. doi: 10.1128/aem.60.3.1006-1013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M. E., Kaiser A., Montville T. J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992 Jul;58(7):2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M. E., Montville T. J. Common mechanistic action of bacteriocins from lactic Acid bacteria. Appl Environ Microbiol. 1993 Sep;59(9):3003–3010. doi: 10.1128/aem.59.9.3003-3010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikindas M. L., García-Garcerá M. J., Driessen A. J., Ledeboer A. M., Nissen-Meyer J., Nes I. F., Abee T., Konings W. N., Venema G. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993 Nov;59(11):3577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D. P., Hutkins R. W. Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl Environ Microbiol. 1992 Oct;58(10):3312–3315. doi: 10.1128/aem.58.10.3312-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcerá M. J., Elferink M. G., Driessen A. J., Konings W. N. In vitro pore-forming activity of the lantibiotic nisin. Role of protonmotive force and lipid composition. Eur J Biochem. 1993 Mar 1;212(2):417–422. doi: 10.1111/j.1432-1033.1993.tb17677.x. [DOI] [PubMed] [Google Scholar]
- Guihard G., Bénédetti H., Besnard M., Letellier L. Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem. 1993 Aug 25;268(24):17775–17780. [PubMed] [Google Scholar]
- Klaenhammer T. R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993 Sep;12(1-3):39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Kordel M., Schüller F., Sahl H. G. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 1989 Feb 13;244(1):99–102. doi: 10.1016/0014-5793(89)81171-8. [DOI] [PubMed] [Google Scholar]
- Ojcius D. M., Young J. D. Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem Sci. 1991 Jun;16(6):225–229. doi: 10.1016/0968-0004(91)90090-i. [DOI] [PubMed] [Google Scholar]
- Okereke A., Montville T. J. Nisin dissipates the proton motive force of the obligate anaerobe Clostridium sporogenes PA 3679. Appl Environ Microbiol. 1992 Aug;58(8):2463–2467. doi: 10.1128/aem.58.8.2463-2467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G., Kordel M., Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol. 1987;149(2):120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- Venema K., Abee T., Haandrikman A. J., Leenhouts K. J., Kok J., Konings W. N., Venema G. Mode of Action of Lactococcin B, a Thiol-Activated Bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993 Apr;59(4):1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel J. K., Ceglowski P., Dobrazański W. T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985 Apr;49(4):969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G., Holo H., Nes I. F., Konings W. N., Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991 Dec;173(24):7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



