Abstract
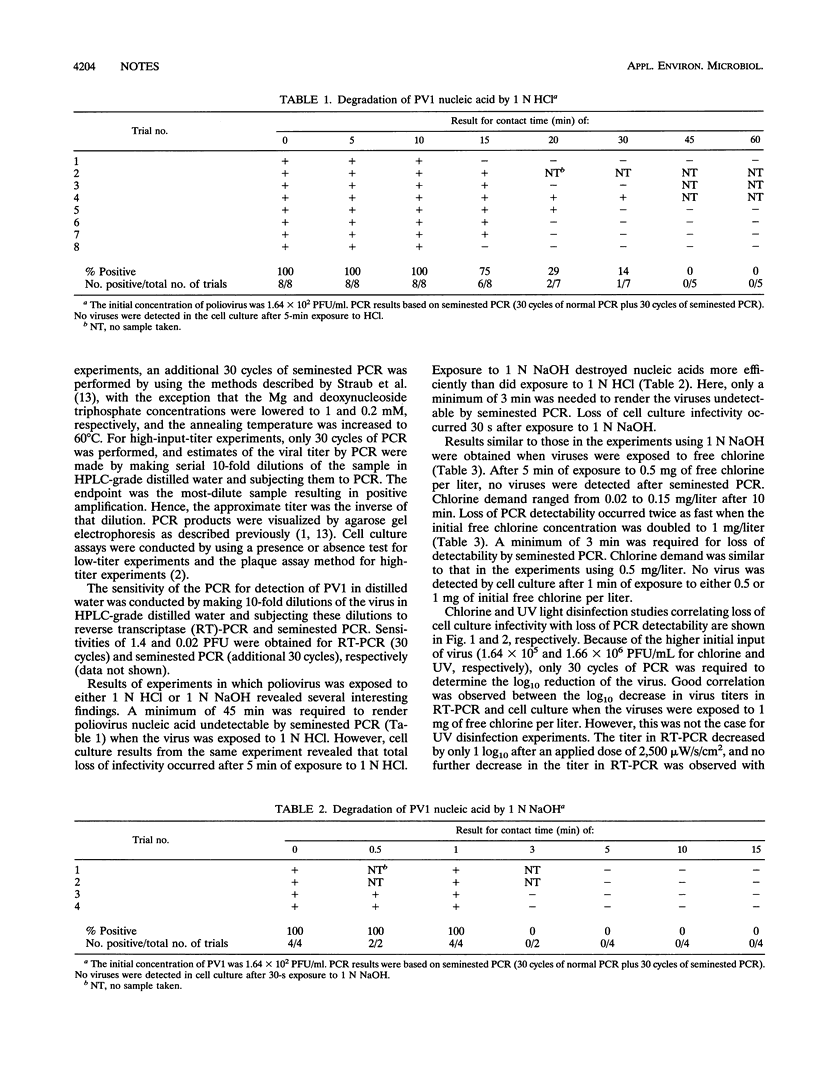
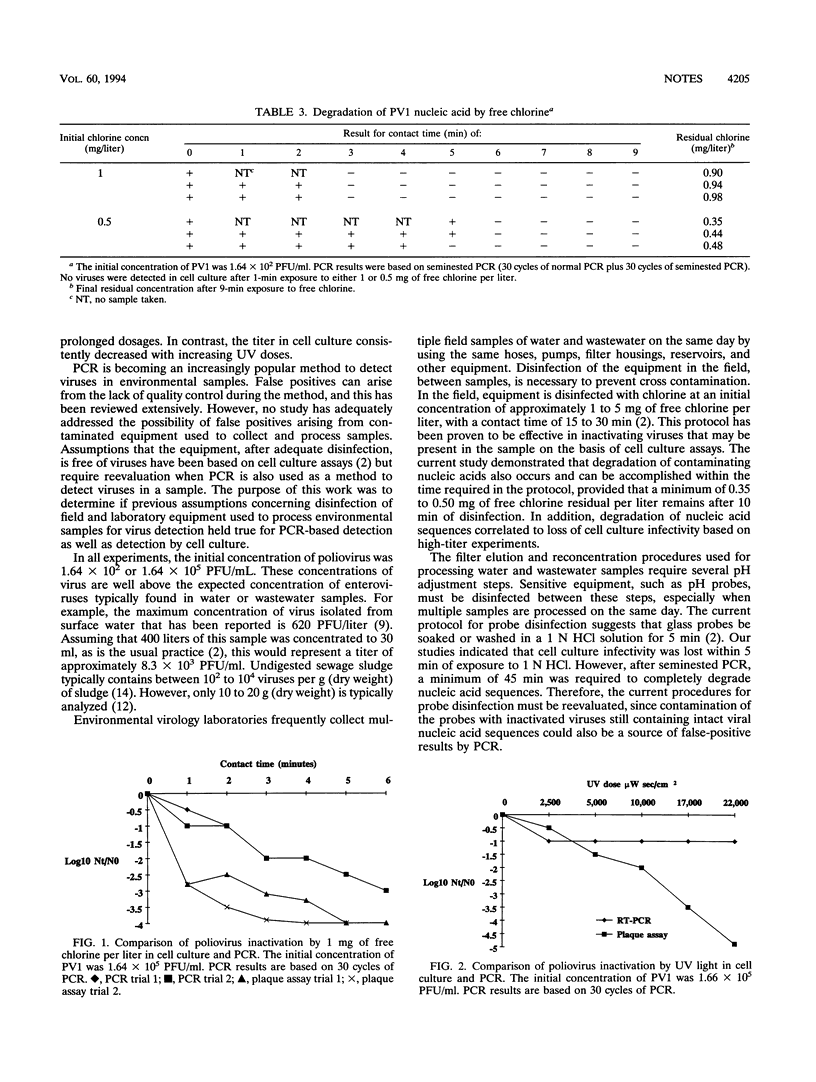
Inactivation of poliovirus type 1 by 1 N HCl, 1 N NaOH, 0.5 and 1.0 mg of free chlorine per liter, and UV light was compared by using cell culture and seminested PCR (30 cycles of reverse transcriptase-PCR plus 30 cycles of seminested PCR). A minimum contact time of 45 min with HCl, 3 min with NaOH, 3 and 6 min with 1.0 and 0.5 mg of free chlorine per liter, respectively, was required to render 1.64 x 10(2) PFU of poliovirus type 1 per ml undetectable by seminested PCR. In cell culture, a minimum contact time of 5 min to HCl, 30 s to NaOH, and 1 min to either chlorine concentration was required to render the viruses undetectable by the plaque assay method. No correlation was observed between results by PCR and cell culture when viruses were exposed to UV light. These data suggest that inactivated virus with intact nucleic acid sequences can be detected by PCR.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszadegan M., Huber M. S., Gerba C. P., Pepper I. L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993 May;59(5):1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. W., Fairfax M. R. Protocol for ultraviolet irradiation of surfaces to reduce PCR contamination. PCR Methods Appl. 1993 Dec;3(3):S15–S17. doi: 10.1101/gr.3.3.s15. [DOI] [PubMed] [Google Scholar]
- Graff J., Ticehurst J., Flehmig B. Detection of hepatitis A virus in sewage sludge by antigen capture polymerase chain reaction. Appl Environ Microbiol. 1993 Oct;59(10):3165–3170. doi: 10.1128/aem.59.10.3165-3170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson K. L., Gerba C. P., Pepper I. L. Polymerase chain reaction detection of nonviable bacterial pathogens. Appl Environ Microbiol. 1993 Oct;59(10):3513–3515. doi: 10.1128/aem.59.10.3513-3515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Dubrou S., Prevot J., Marechal J., López-Pila J. M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl Environ Microbiol. 1993 Apr;59(4):1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. F., Naranjo J., Gerba C. P. Evaluation of MK filters for recovery of enteroviruses from tap water. Appl Environ Microbiol. 1994 Jun;60(6):1974–1977. doi: 10.1128/aem.60.6.1974-1977.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub T. M., Pepper I. L., Abbaszadegan M., Gerba C. P. A method to detect enteroviruses in sewage sludge-amended soil using the PCR. Appl Environ Microbiol. 1994 Mar;60(3):1014–1017. doi: 10.1128/aem.60.3.1014-1017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub T. M., Pepper I. L., Gerba C. P. Hazards from pathogenic microorganisms in land-disposed sewage sludge. Rev Environ Contam Toxicol. 1993;132:55–91. doi: 10.1007/978-1-4684-7065-9_3. [DOI] [PubMed] [Google Scholar]



