Abstract
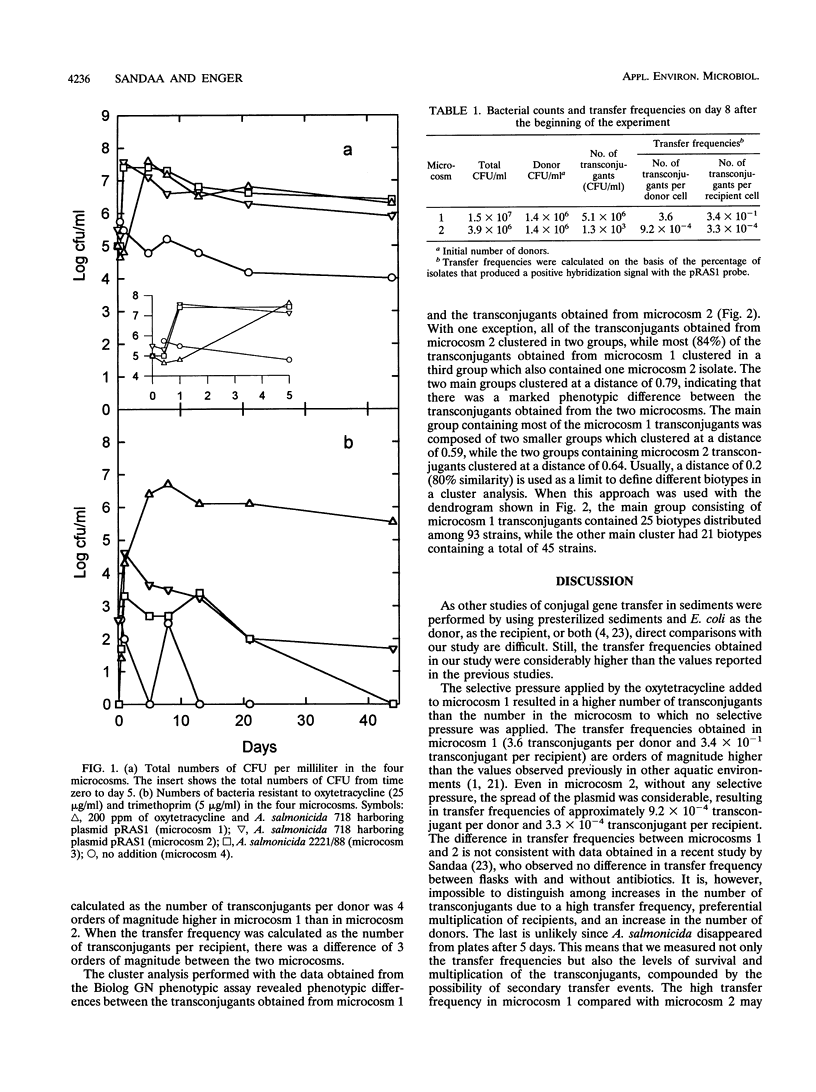
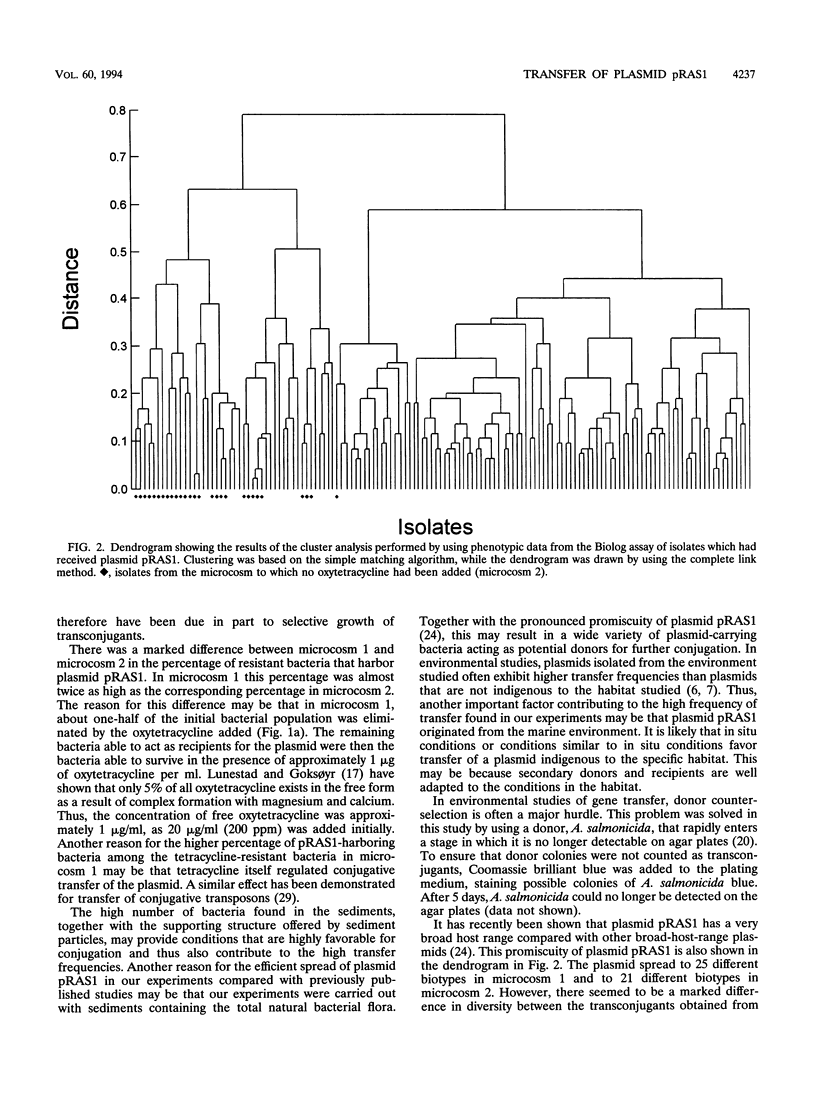
The results of microcosm experiments performed with the fish-pathogenic bacterium Aeromonas salmonicida acting as a donor showed that promiscuous plasmid pRAS1, which encodes tetracycline resistance, is transferred at a high frequency in marine sediments even in the absence of a selective factor. The presence of oxytetracycline resulted in an increase in the transfer frequency compared with that of a microcosm to which no selective factor was added. Transfer frequencies of 3.4 × 10-1 transconjugant per recipient and 3.6 transconjugants per donor cell were obtained in a microcosm to which oxytetracycline had been added. Hybridization with a DNA probe specific for plasmid pRAS1 revealed that 45.8% of the oxytetracycline-resistant isolates obtained from a microcosm with no selective pressure carried the plasmid, while 86.8% of the isolates obtained from a microcosm to which oxytetracycline had been added carried the plasmid. Phenotypic characterization of the transconjugants revealed that the plasmid had been transferred to a variety of different biotypes in both microcosms. The diversity among the transconjugants isolated from the microcosm to which oxytetracycline had been added was substantially lower than the diversity among the transconjugants isolated from the microcosm to which no selective agent had been added.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale M. J., Fry J. C., Day M. J. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J Gen Microbiol. 1987 Nov;133(11):3099–3107. doi: 10.1099/00221287-133-11-3099. [DOI] [PubMed] [Google Scholar]
- Bale M. J., Fry J. C., Day M. J. Transfer and occurrence of large mercury resistance plasmids in river epilithon. Appl Environ Microbiol. 1988 Apr;54(4):972–978. doi: 10.1128/aem.54.4.972-978.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baya A. M., Brayton P. R., Brown V. L., Grimes D. J., Russek-Cohen E., Colwell R. R. Coincident plasmids and antimicrobial resistance in marine bacteria isolated from polluted and unpolluted Atlantic Ocean samples. Appl Environ Microbiol. 1986 Jun;51(6):1285–1292. doi: 10.1128/aem.51.6.1285-1292.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breittmayer V. A., Gauthier M. J. Influence of glycine betaine on the transfer of plasmid RP4 between Escherichia coli strains in marine sediments. Lett Appl Microbiol. 1990 Feb;10(2):65–68. doi: 10.1111/j.1472-765x.1990.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Buck J. D. Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl Environ Microbiol. 1982 Oct;44(4):992–993. doi: 10.1128/aem.44.4.992-993.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealt M. A., Chai M. D., Alpert K. B., Boyer J. C. Transfer of plasmids pBR322 and pBR325 in wastewater from laboratory strains of Escherichia coli to bacteria indigenous to the waste disposal system. Appl Environ Microbiol. 1985 Apr;49(4):836–841. doi: 10.1128/aem.49.4.836-841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner F. J., Chatterjee P., Barkay T., Bourquin A. W. Capacity of aquatic bacteria to act as recipients of plasmid DNA. Appl Environ Microbiol. 1988 Jan;54(1):115–117. doi: 10.1128/aem.54.1.115-117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. E., Hild E., Marshall K. C., Hermansson M. Conjugative Plasmid Transfer between Bacteria under Simulated Marine Oligotrophic Conditions. Appl Environ Microbiol. 1993 Apr;59(4):1035–1040. doi: 10.1128/aem.59.4.1035-1040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada H. S., Sizemore R. K. Incidence of Plasmids in Marine Vibrio spp. Isolated from an Oil Field in the Northwestern Gulf of Mexico. Appl Environ Microbiol. 1981 Jan;41(1):199–202. doi: 10.1128/aem.41.1.199-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson M., Jones G. W., Kjelleberg S. Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria of the marine air-water interface. Appl Environ Microbiol. 1987 Oct;53(10):2338–2342. doi: 10.1128/aem.53.10.2338-2342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach P. A., Grimes D. J. R-plasmid transfer in a wastewater treatment plant. Appl Environ Microbiol. 1982 Dec;44(6):1395–1403. doi: 10.1128/aem.44.6.1395-1403.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini P., Fertels S., Nave D., Gealt M. A. Mobilization of plasmid pHSV106 from Escherichia coli HB101 in a laboratory-scale waste treatment facility. Appl Environ Microbiol. 1987 Apr;53(4):665–671. doi: 10.1128/aem.53.4.665-671.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Morchoe S. B., Ogunseitan O., Sayler G. S., Miller R. V. Conjugal transfer of R68.45 and FP5 between Pseudomonas aeruginosa strains in a freshwater environment. Appl Environ Microbiol. 1988 Aug;54(8):1923–1929. doi: 10.1128/aem.54.8.1923-1929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore R. K., Colwell R. R. Plasmids carried by antibiotic-resistant marine bacteria. Antimicrob Agents Chemother. 1977 Sep;12(3):373–382. doi: 10.1128/aac.12.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Incidence of R + Escherichia coli in coastal bathing waters of Britain. Nature. 1971 Nov 19;234(5325):155–156. doi: 10.1038/234155a0. [DOI] [PubMed] [Google Scholar]
- Stevens A. M., Shoemaker N. B., Li L. Y., Salyers A. A. Tetracycline regulation of genes on Bacteroides conjugative transposons. J Bacteriol. 1993 Oct;175(19):6134–6141. doi: 10.1128/jb.175.19.6134-6141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



