Abstract
Previous work has shown that attachment of Vibrio harveyi to chitin is specific and involves at least two chitin-binding peptides. However, the roles and regulation of these chitin-binding peptides in attachment are still unclear. Here we show that preincubation with the oligomeric sugars composing chitin stimulated chitinase activity, cellular attachment to chitin, and production of chitin-binding peptides. One of these peptides, a 53-kDa peptide, is produced constitutively and appears to mediate initial attachment to chitin. Synthesis of another peptide, a 150-kDa chitin-binding peptide, is induced by chitin and thus may be involved in time-dependent attachment. Coordinated regulation of attachment and degradation of chitin may give bacteria like V. harveyi a selective advantage over other bacteria in nutrient-poor aquatic environments.
Full text
PDF
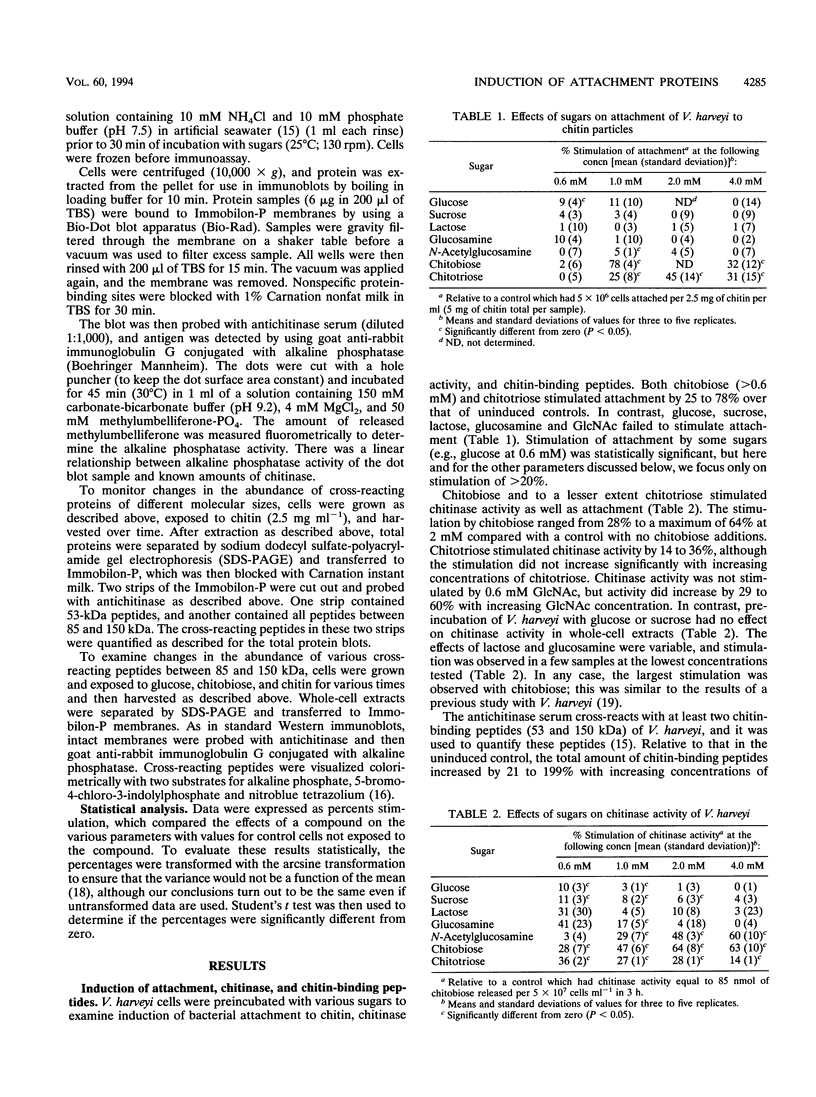
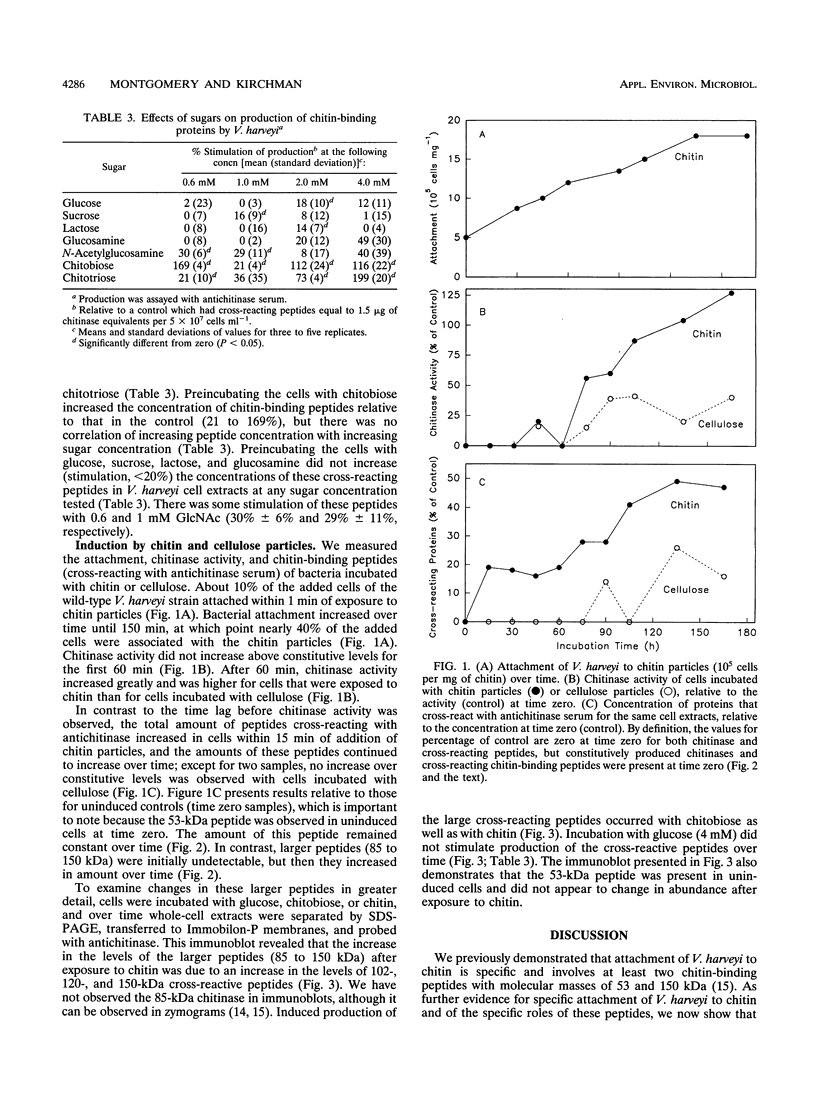
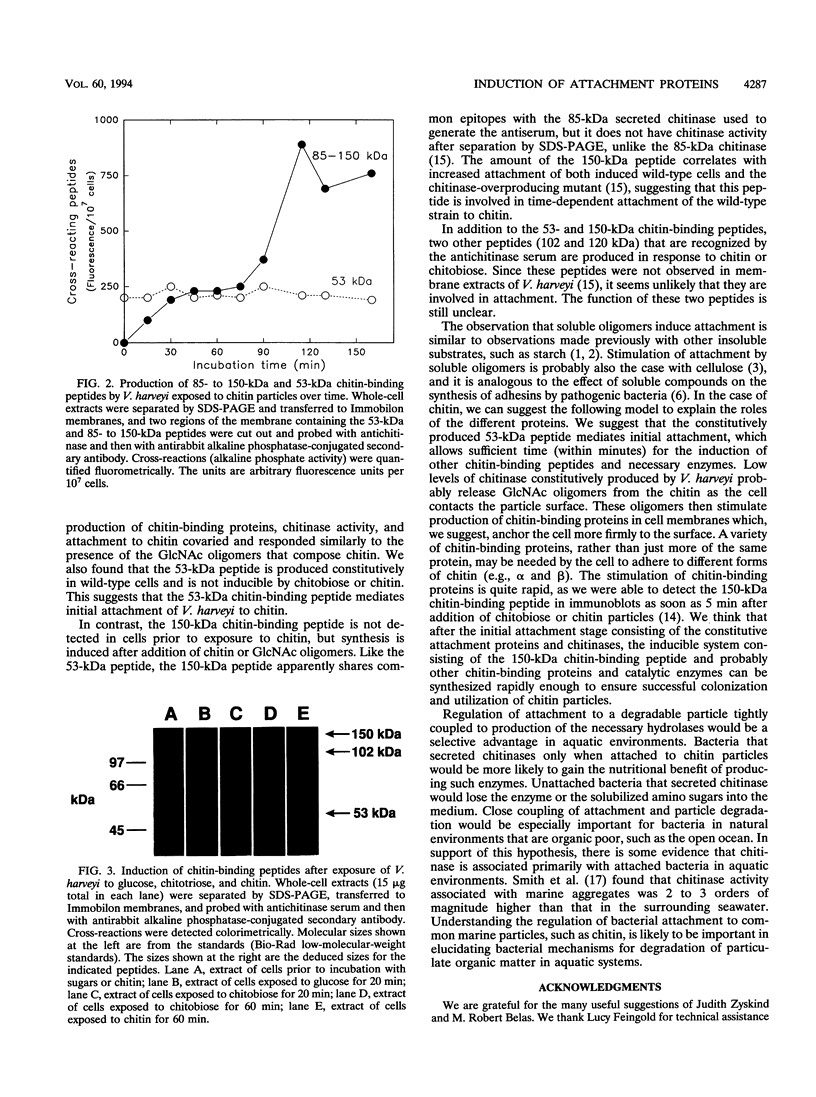

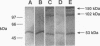
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Salyers A. A. Biochemical evidence that starch breakdown by Bacteroides thetaiotaomicron involves outer membrane starch-binding sites and periplasmic starch-degrading enzymes. J Bacteriol. 1989 Jun;171(6):3192–3198. doi: 10.1128/jb.171.6.3192-3198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. L., Salyers A. A. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1989 Jun;171(6):3199–3204. doi: 10.1128/jb.171.6.3199-3204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Mileham A., Simon M., Silverman M. Transposon mutagenesis of marine Vibrio spp. J Bacteriol. 1984 Jun;158(3):890–896. doi: 10.1128/jb.158.3.890-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. S., Apostol J. M., Jr, Fullner K. J., Moseley S. L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993 Mar;7(6):993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Béguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Mooi F. R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- Göransson M., Sondén B., Nilsson P., Dagberg B., Forsman K., Emanuelsson K., Uhlin B. E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- Hacker J. Role of fimbrial adhesins in the pathogenesis of Escherichia coli infections. Can J Microbiol. 1992 Jul;38(7):720–727. doi: 10.1139/m92-118. [DOI] [PubMed] [Google Scholar]
- Hobbs M., Collie E. S., Free P. D., Livingston S. P., Mattick J. S. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1993 Mar;7(5):669–682. doi: 10.1111/j.1365-2958.1993.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S. H., Greene R. V., Griffin H. L. Adhesive properties of a symbiotic bacterium from a wood-boring marine shipworm. Appl Environ Microbiol. 1990 May;56(5):1317–1322. doi: 10.1128/aem.56.5.1317-1322.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue O. J., Lee J. H., Isaacson R. E. Transcriptional organization of the Escherichia coli pilus adhesin K99. Mol Microbiol. 1993 Nov;10(3):607–613. doi: 10.1111/j.1365-2958.1993.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Lamed R., Naimark J., Morgenstern E., Bayer E. A. Specialized cell surface structures in cellulolytic bacteria. J Bacteriol. 1987 Aug;169(8):3792–3800. doi: 10.1128/jb.169.8.3792-3800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery M. T., Kirchman D. L. Role of Chitin-Binding Proteins in the Specific Attachment of the Marine Bacterium Vibrio harveyi to Chitin. Appl Environ Microbiol. 1993 Feb;59(2):373–379. doi: 10.1128/aem.59.2.373-379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M., Colwell R. R. A rapid test for chitinase activity that uses 4-methylumbelliferyl-N-acetyl-beta-D-glucosaminide. Appl Environ Microbiol. 1987 Jul;53(7):1718–1720. doi: 10.1128/aem.53.7.1718-1720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude M. W., Braaten B. A., Low D. A. Evidence for global regulatory control of pilus expression in Escherichia coli by Lrp and DNA methylation: model building based on analysis of pap. Mol Microbiol. 1992 Sep;6(17):2429–2435. doi: 10.1111/j.1365-2958.1992.tb01418.x. [DOI] [PubMed] [Google Scholar]