Abstract
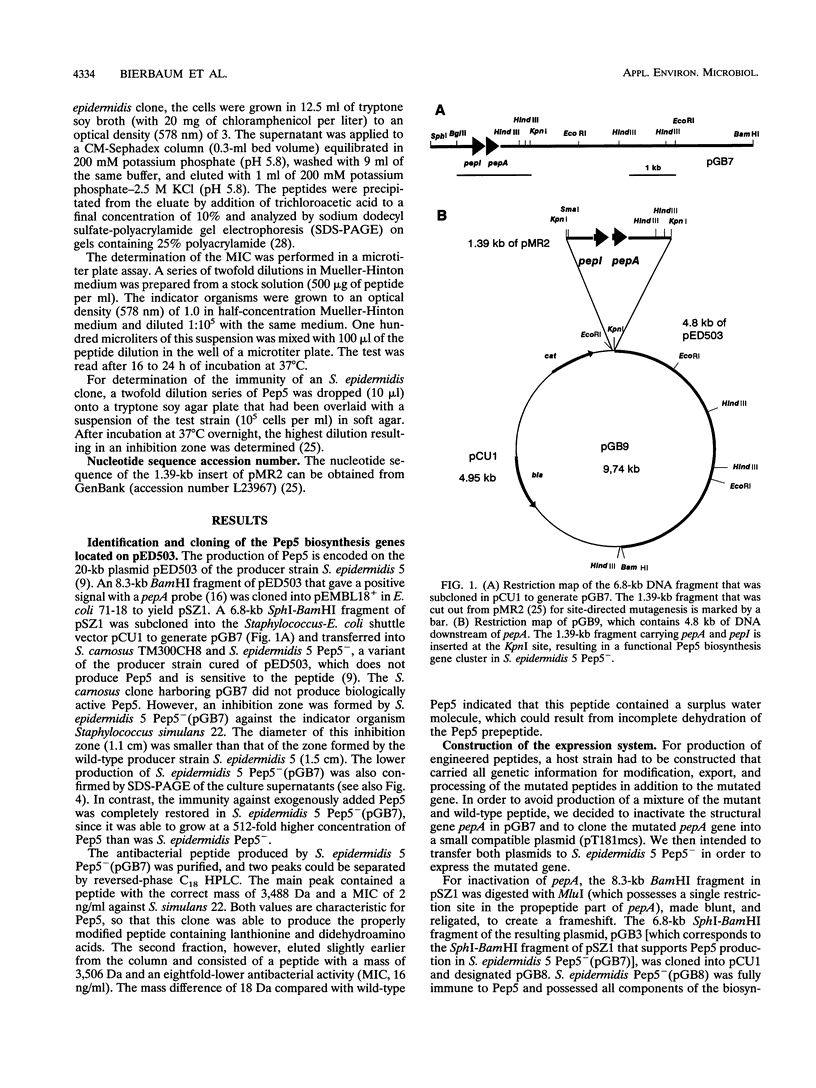
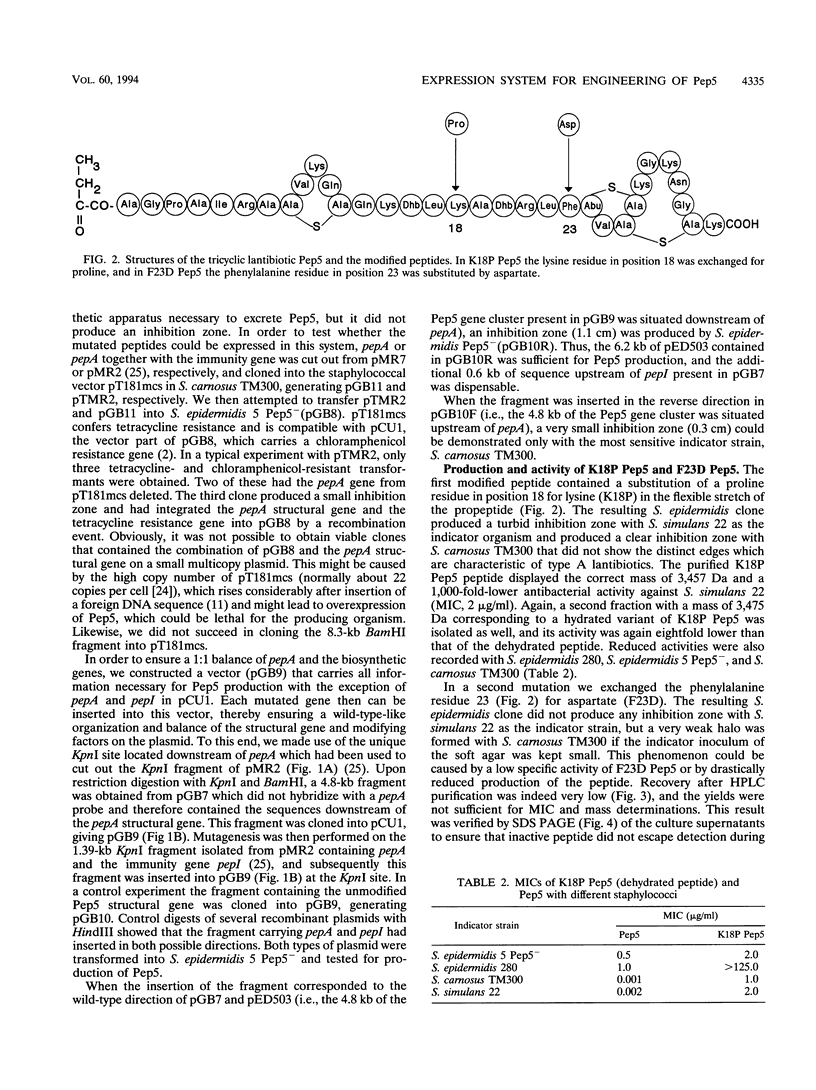
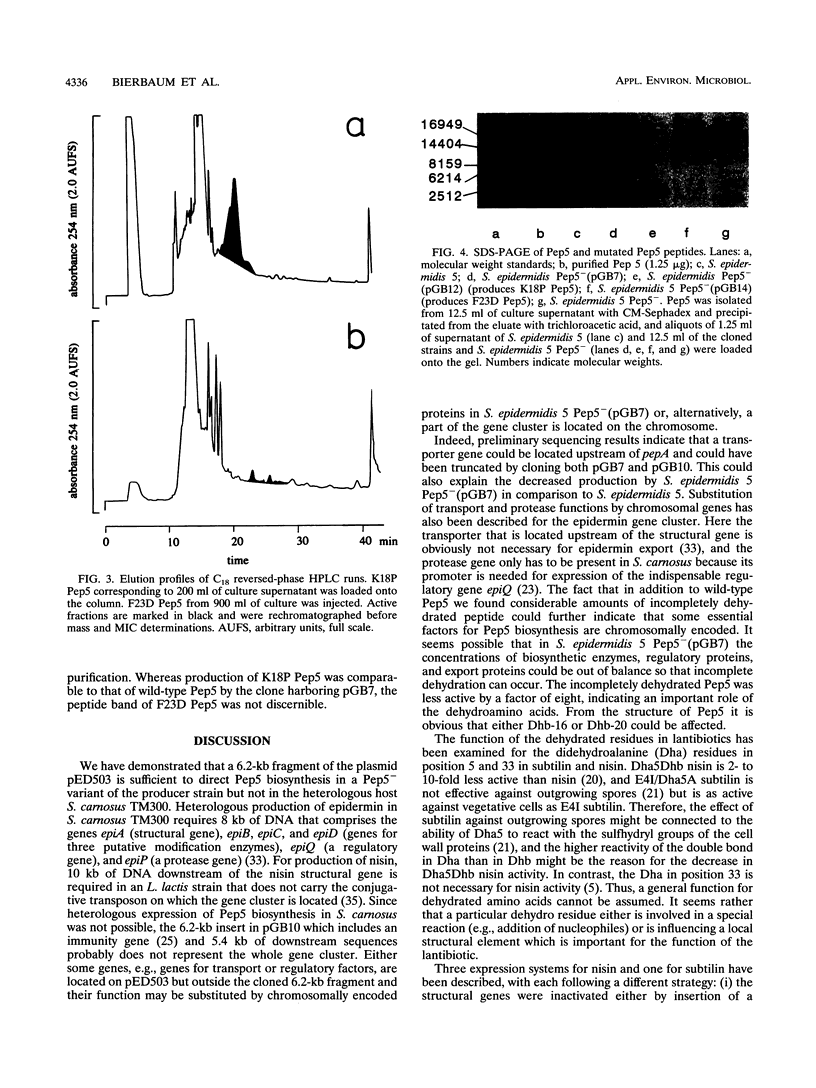
Pep5 is a lanthionine-containing antimicrobial peptide which is produced by Staphylococcus epidermidis 5. Its structural gene, pepA, is located on the 20-kb plasmid pED503. A 6.2-kb fragment of pED503 containing pepA, the immunity gene pepI, and 5.4 kb of downstream sequence was able to direct biosynthesis of biologically active Pep5 in a nonproducing variant of the producer strain which is devoid of pED503. In addition to producing wild-type Pep5 with a molecular mass of 3,488 Da, the clone produced a peptide with an eightfold-lower bactericidal activity and a mass of 3,506 Da, indicative of incomplete dehydration of one hydroxyamino acid. For construction of the expression system, this 6.2-kb fragment was cut into a 1.39-kb fragment containing pepA and pepI and a 4.8-kb fragment covering the remaining downstream region. This 4.8-kb fragment was directly cloned into an Escherichia coli-Staphylococcus shuttle vector, yielding a new plasmid (pGB9) into which mutated pepA genes generated on the 1.39-kb fragment can be reinserted to yield a functional Pep5 biosynthesis gene cluster. To test the expression system, two mutants were constructed. Lys-18-Pro Pep5 was produced in its dehydrated form and a partially hydrated form in amounts comparable to those of the wild-type peptide. In contrast, only small amounts of Phe-23-Asp Pep5 were excreted, indicating that some residues in the propeptide part of the prelantibiotic may be crucial for certain steps in the biosynthetic pathway of lantibiotics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin J., Götz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Augustin J., Rosenstein R., Wieland B., Schneider U., Schnell N., Engelke G., Entian K. D., Götz F. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur J Biochem. 1992 Mar 15;204(3):1149–1154. doi: 10.1111/j.1432-1033.1992.tb16740.x. [DOI] [PubMed] [Google Scholar]
- Bierbaum G., Sahl H. G. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-L-alanine amidase. J Bacteriol. 1987 Dec;169(12):5452–5458. doi: 10.1128/jb.169.12.5452-5458.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. J., Hansen J. N. Determination of the sequence of spaE and identification of a promoter in the subtilin (spa) operon in Bacillus subtilis. J Bacteriol. 1992 Oct;174(20):6699–6702. doi: 10.1128/jb.174.20.6699-6702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Hao Z., Gasson M. J. A lactococcal expression system for engineered nisins. Appl Environ Microbiol. 1992 Nov;58(11):3683–3693. doi: 10.1128/aem.58.11.3683-3693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersfeld-Dressen H., Sahl H. G., Brandis H. Plasmid involvement in production of and immunity to the staphylococcin-like peptide Pep 5. J Gen Microbiol. 1984 Nov;130(11):3029–3035. doi: 10.1099/00221287-130-11-3029. [DOI] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D., Kellner R., Jung G., Reis M., Sahl H. G. Pep5, a new lantibiotic: structural gene isolation and prepeptide sequence. Arch Microbiol. 1989;152(1):16–19. doi: 10.1007/BF00447005. [DOI] [PubMed] [Google Scholar]
- Klein C., Kaletta C., Entian K. D. Biosynthesis of the lantibiotic subtilin is regulated by a histidine kinase/response regulator system. Appl Environ Microbiol. 1993 Jan;59(1):296–303. doi: 10.1128/aem.59.1.296-303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers O. P., Beerthuyzen M. M., Siezen R. J., De Vos W. M. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem. 1993 Aug 15;216(1):281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- Kuipers O. P., Rollema H. S., Yap W. M., Boot H. J., Siezen R. J., de Vos W. M. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J Biol Chem. 1992 Dec 5;267(34):24340–24346. [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Enhancement of the chemical and antimicrobial properties of subtilin by site-directed mutagenesis. J Biol Chem. 1992 Dec 15;267(35):25078–25085. [PubMed] [Google Scholar]
- Peschel A., Augustin J., Kupke T., Stevanovic S., Götz F. Regulation of epidermin biosynthetic genes by EpiQ. Mol Microbiol. 1993 Jul;9(1):31–39. doi: 10.1111/j.1365-2958.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Projan S. J., Carleton S., Novick R. P. Determination of plasmid copy number by fluorescence densitometry. Plasmid. 1983 Mar;9(2):182–190. doi: 10.1016/0147-619x(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Reis M., Eschbach-Bludau M., Iglesias-Wind M. I., Kupke T., Sahl H. G. Producer immunity towards the lantibiotic Pep5: identification of the immunity gene pepI and localization and functional analysis of its gene product. Appl Environ Microbiol. 1994 Aug;60(8):2876–2883. doi: 10.1128/aem.60.8.2876-2883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Production, purification and chemical properties of an antistaphylococcal agent produced by Staphylococcus epidermidis. J Gen Microbiol. 1981 Dec;127(2):377–384. doi: 10.1099/00221287-127-2-377. [DOI] [PubMed] [Google Scholar]
- Sahl H. G., Grossgarten M., Widger W. R., Cramer W. A., Brandis H. Structural similarities of the staphylococcin-like peptide Pep-5 to the peptide antibiotic nisin. Antimicrob Agents Chemother. 1985 May;27(5):836–840. doi: 10.1128/aac.27.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G., Kordel M., Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol. 1987;149(2):120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Engelke G., Augustin J., Rosenstein R., Ungermann V., Götz F., Entian K. D. Analysis of genes involved in the biosynthesis of lantibiotic epidermin. Eur J Biochem. 1992 Feb 15;204(1):57–68. doi: 10.1111/j.1432-1033.1992.tb16605.x. [DOI] [PubMed] [Google Scholar]
- Schnell N., Entian K. D., Schneider U., Götz F., Zähner H., Kellner R., Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988 May 19;333(6170):276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wade D., Andreu D., Mitchell S. A., Silveira A. M., Boman A., Boman H. G., Merrifield R. B. Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res. 1992 Nov;40(5):429–436. doi: 10.1111/j.1399-3011.1992.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Weil H. P., Beck-Sickinger A. G., Metzger J., Stevanovic S., Jung G., Josten M., Sahl H. G. Biosynthesis of the lantibiotic Pep5. Isolation and characterization of a prepeptide containing dehydroamino acids. Eur J Biochem. 1990 Nov 26;194(1):217–223. doi: 10.1111/j.1432-1033.1990.tb19446.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zell R., Fritz H. J. DNA mismatch-repair in Escherichia coli counteracting the hydrolytic deamination of 5-methyl-cytosine residues. EMBO J. 1987 Jun;6(6):1809–1815. doi: 10.1002/j.1460-2075.1987.tb02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. R., Polman J., Beerthuyzen M. M., Siezen R. J., Kuipers O. P., De Vos W. M. Characterization of the Lactococcus lactis nisin A operon genes nisP, encoding a subtilisin-like serine protease involved in precursor processing, and nisR, encoding a regulatory protein involved in nisin biosynthesis. J Bacteriol. 1993 May;175(9):2578–2588. doi: 10.1128/jb.175.9.2578-2588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




