Abstract
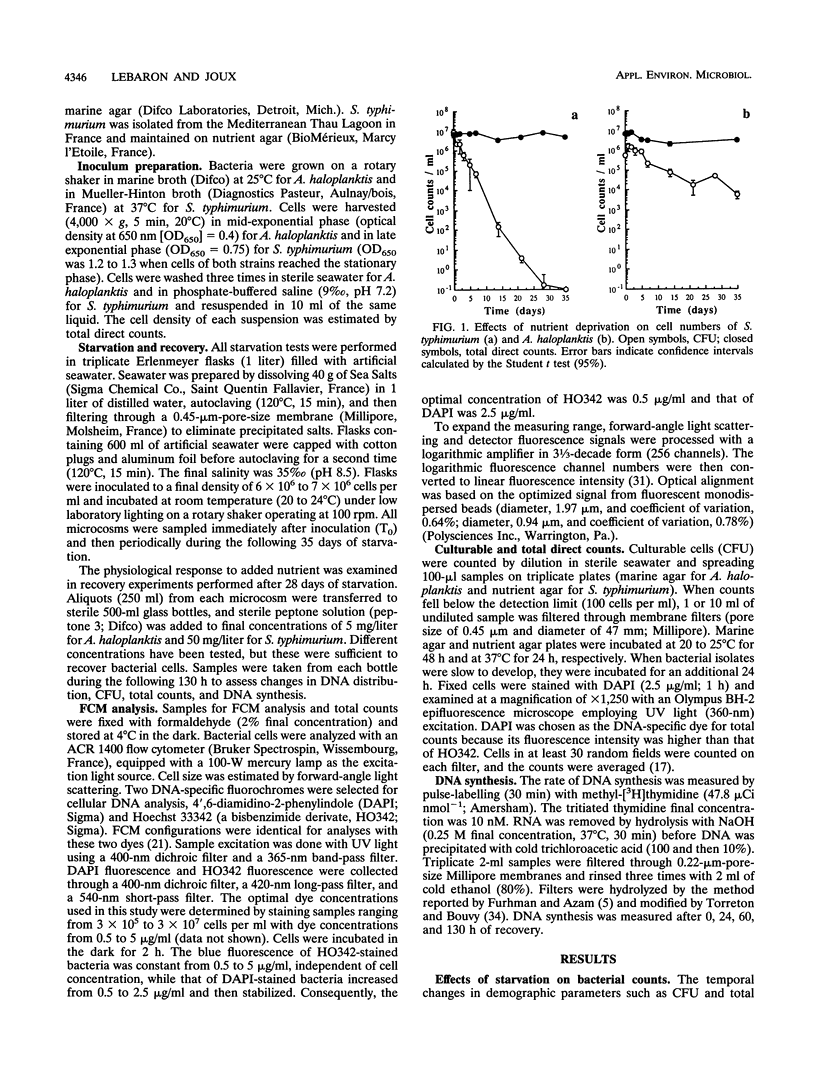
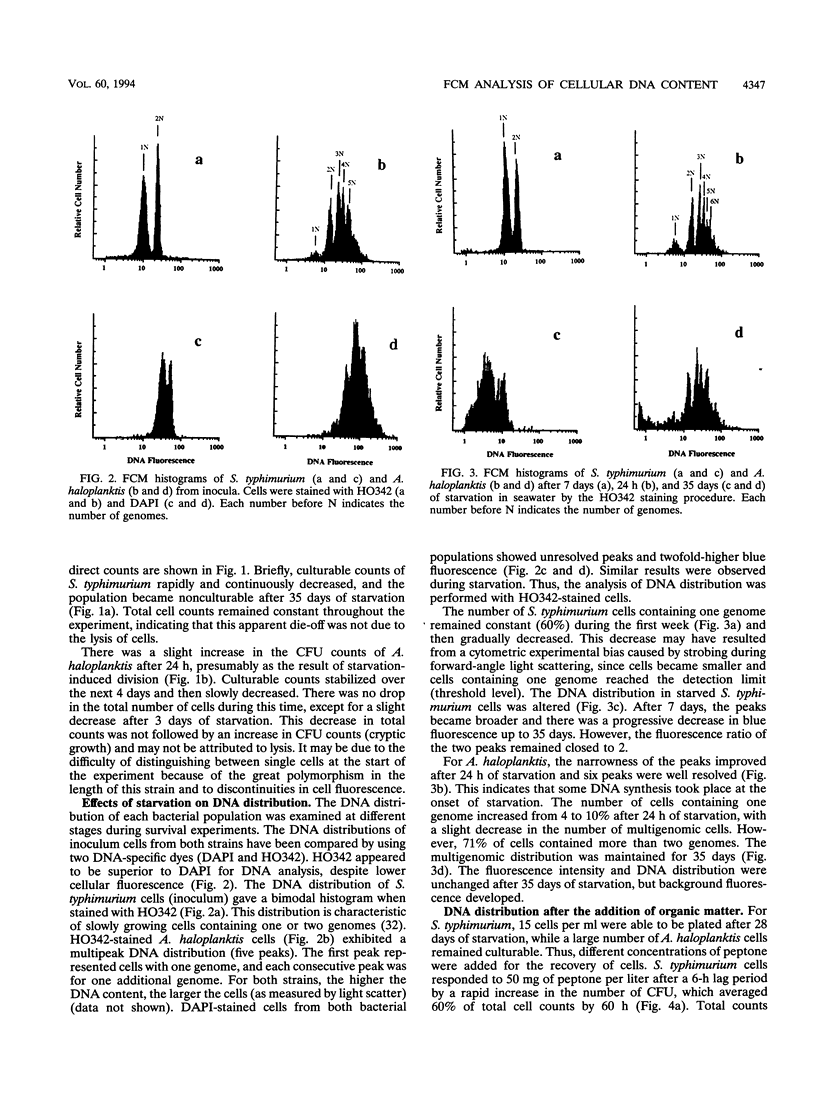
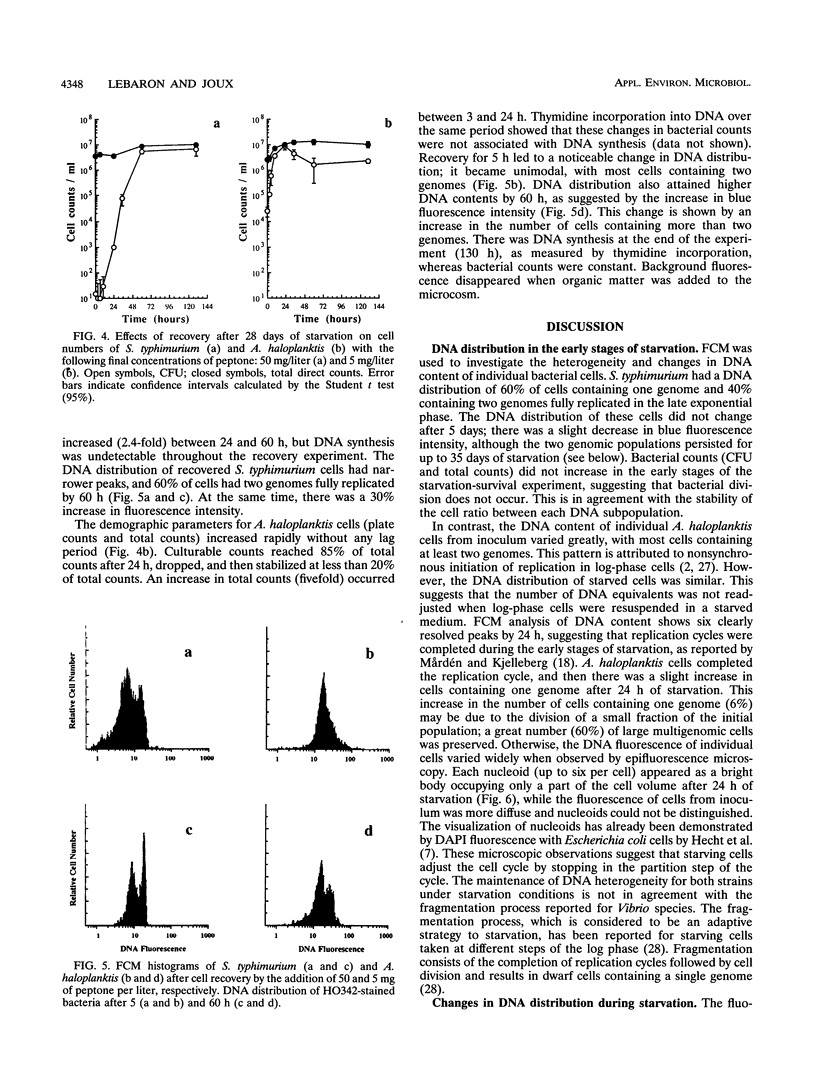
Flow cytometry was used to investigate the heterogeneity of the DNA content of Salmonella typhimurium and Alteromonas haloplanktis cells that were starved and allowed to recover in seawater. Hoechst 33342 (bisbenzimide) was used as a DNA-specific dye to discriminate between DNA subpopulations. The DNA contents of both strains were heterogeneous during starvation. S. typhimurium cells contained one or two genomes, and A. haloplanktis cells contained up to six genomes. S. typhimurium genomes were fully replicated at the onset of starvation. Each replication cycle was completed in the early stage of starvation for A. haloplanktis by stopping cells in the partition step of the cell cycle prior to division. Multigenomic marine cells can undergo rapid cell division without DNA synthesis upon recovery, resulting in large fluctuations in the DNA contents of individual cells. In contrast, the heterogeneity of the DNA distribution of S. typhimurium cells was preserved during recovery. The fluctuations in the DNA fluorescence of this strain seem to be due to topological changes in DNA. Flow cytometry may provide a new approach to understanding dynamic and physiological changes in bacteria by detecting cellular heterogeneity in response to different growth conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boye E., Løbner-Olesen A. Bacterial growth control studied by flow cytometry. Res Microbiol. 1991 Feb-Apr;142(2-3):131–135. doi: 10.1016/0923-2508(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Brauns L. A., Hudson M. C., Oliver J. D. Use of the polymerase chain reaction in detection of culturable and nonculturable Vibrio vulnificus cells. Appl Environ Microbiol. 1991 Sep;57(9):2651–2655. doi: 10.1128/aem.57.9.2651-2655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. L. Uptake and incorporation of thymidine by bacterial isolates from an upwelling environment. Appl Environ Microbiol. 1989 May;55(5):1267–1272. doi: 10.1128/aem.55.5.1267-1272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. J., Labedan B., Breittmayer V. A. Influence of DNA supercoiling on the loss of culturability of Escherichia coli cells incubated in seawater. Mol Ecol. 1992 Oct;1(3):183–190. doi: 10.1111/j.1365-294x.1992.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Hecht R. M., Taggart R. T., Pettijohn D. E. Size and DNA content of purfied E. coli nucleoids observed by fluorencence microscopy. Nature. 1975 Jan 3;253(5486):60–62. doi: 10.1038/253060a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Hoff K. A. Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl Environ Microbiol. 1989 Jul;55(7):1775–1786. doi: 10.1128/aem.55.7.1775-1786.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L. S., Rouviere-Yaniv J., Drlica K. Bacterial DNA supercoiling and [ATP]/[ADP] ratio: changes associated with salt shock. J Bacteriol. 1991 Jun;173(12):3914–3917. doi: 10.1128/jb.173.12.3914-3917.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprelyants A. S., Kell D. B. Dormancy in Stationary-Phase Cultures of Micrococcus luteus: Flow Cytometric Analysis of Starvation and Resuscitation. Appl Environ Microbiol. 1993 Oct;59(10):3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Kramer J. G., Singleton F. L. Variations in rRNA content of marine Vibrio spp. during starvation-survival and recovery. Appl Environ Microbiol. 1992 Jan;58(1):201–207. doi: 10.1128/aem.58.1.201-207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbies M. Flow cytometric recognition of clastogen induced chromatin damage in G0/G1 lymphocytes by non-stoichiometric Hoechst fluorochrome binding. Cytometry. 1990;11(3):386–394. doi: 10.1002/cyto.990110309. [DOI] [PubMed] [Google Scholar]
- Morgan J. A., Rhodes G., Pickup R. W. Survival of nonculturable Aeromonas salmonicida in lake water. Appl Environ Microbiol. 1993 Mar;59(3):874–880. doi: 10.1128/aem.59.3.874-880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moré M. I., Herrick J. B., Silva M. C., Ghiorse W. C., Madsen E. L. Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Environ Microbiol. 1994 May;60(5):1572–1580. doi: 10.1128/aem.60.5.1572-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer C. L., Morita R. Y. Effect of growth rate and starvation-survival on cellular DNA, RNA, and protein of a psychrophilic marine bacterium. Appl Environ Microbiol. 1989 Oct;55(10):2710–2716. doi: 10.1128/aem.55.10.2710-2716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne C. P., Ní Bhriain N., Dorman C. J. The DNA supercoiling-sensitive expression of the Salmonella typhimurium his operon requires the his attenuator and is modulated by anaerobiosis and by osmolarity. Mol Microbiol. 1992 Sep;6(17):2467–2476. doi: 10.1111/j.1365-2958.1992.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Sandhu L. C., Warters R. L., Dethlefsen L. A. Fluorescence studies of Hoechst 33342 with supercoiled and relaxed plasmid pBR322 DNA. Cytometry. 1985 May;6(3):191–194. doi: 10.1002/cyto.990060304. [DOI] [PubMed] [Google Scholar]
- Schmid I., Schmid P., Giorgi J. V. Conversion of logarithmic channel numbers into relative linear fluorescence intensity. Cytometry. 1988 Nov;9(6):533–538. doi: 10.1002/cyto.990090605. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Boye E. Bacterial growth studied by flow cytometry. Cytometry. 1980 Jul;1(1):32–36. doi: 10.1002/cyto.990010108. [DOI] [PubMed] [Google Scholar]
- Thorsen B. K., Enger O., Norland S., Hoff K. A. Long-term starvation survival of Yersinia ruckeri at different salinities studied by microscopical and flow cytometric methods. Appl Environ Microbiol. 1992 May;58(5):1624–1628. doi: 10.1128/aem.58.5.1624-1628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]




