Abstract
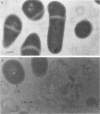
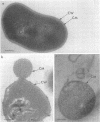
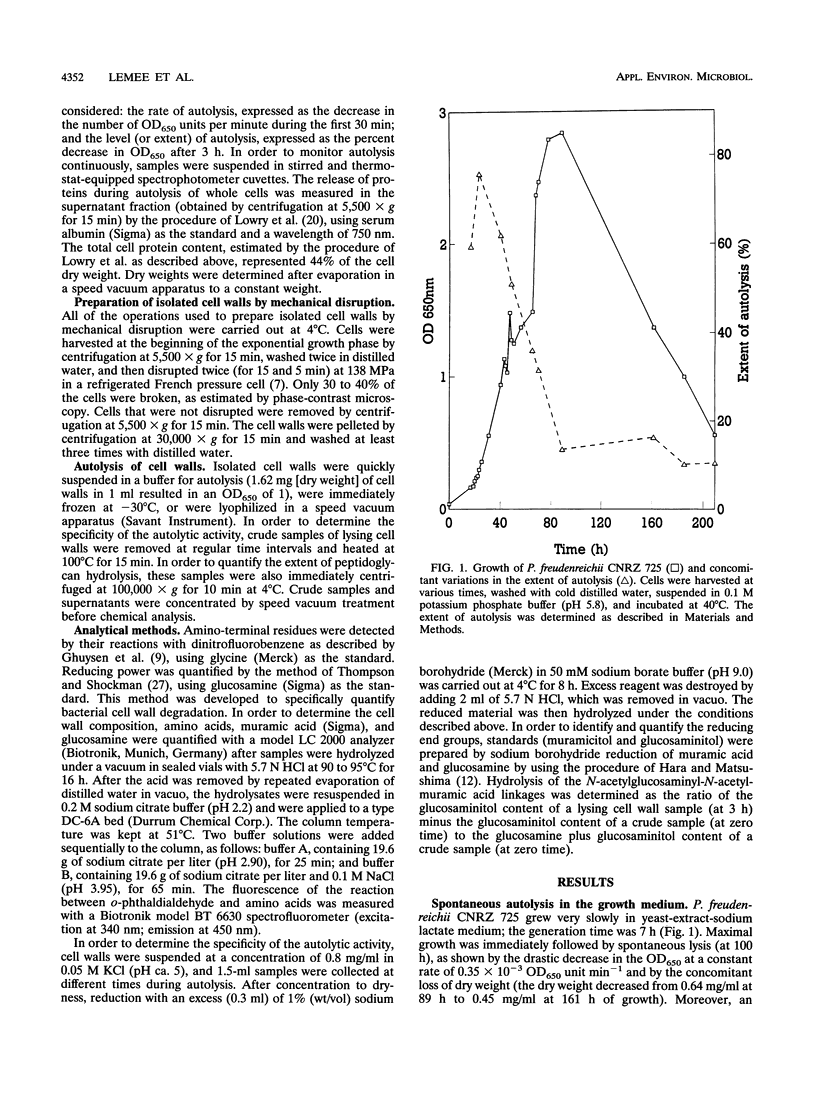
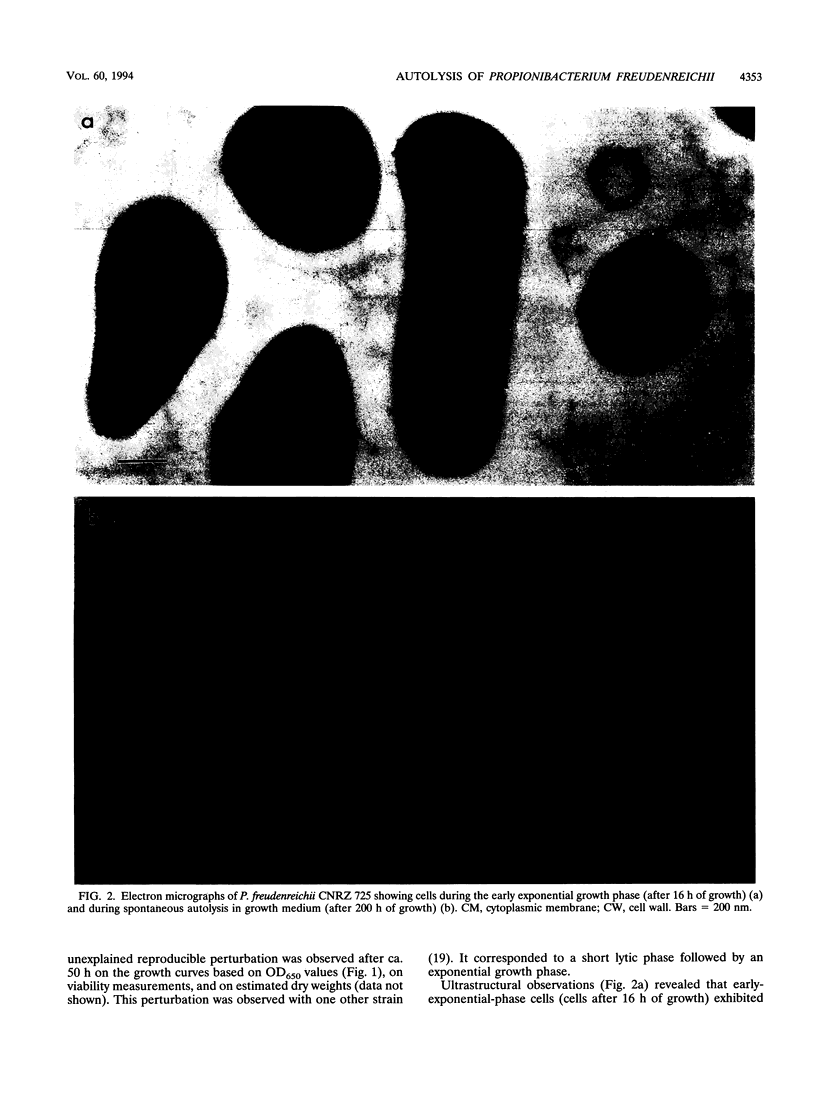
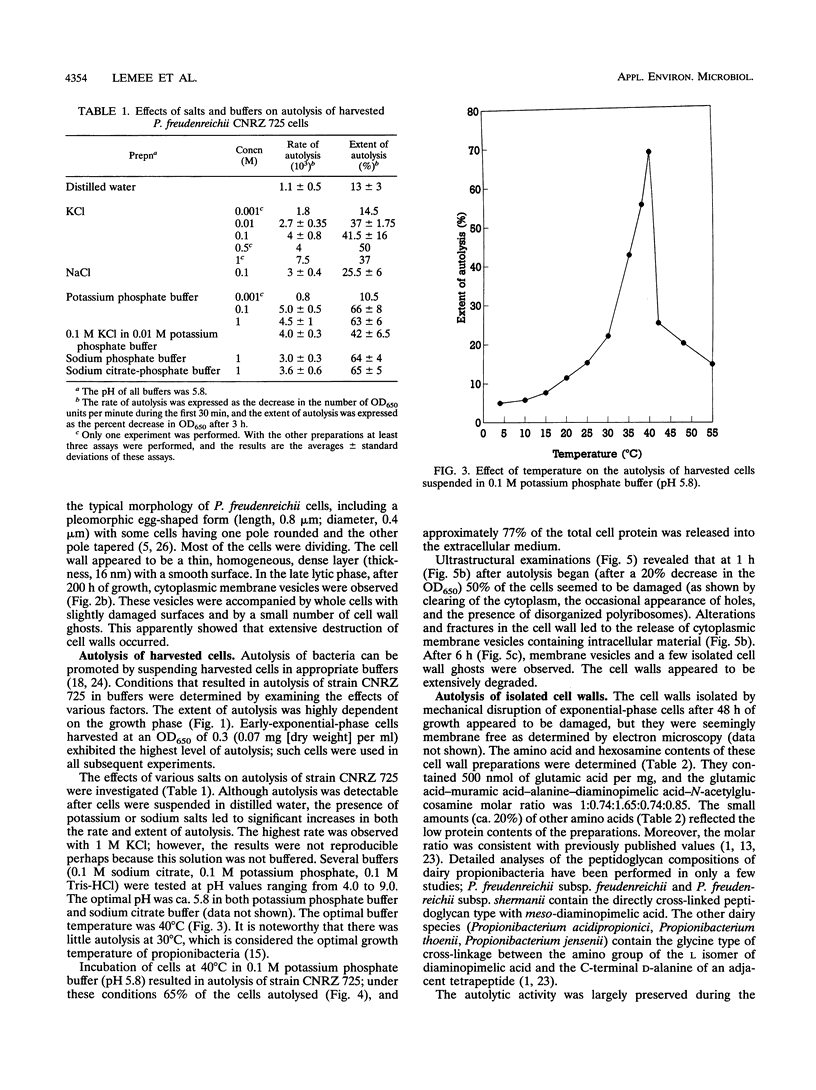
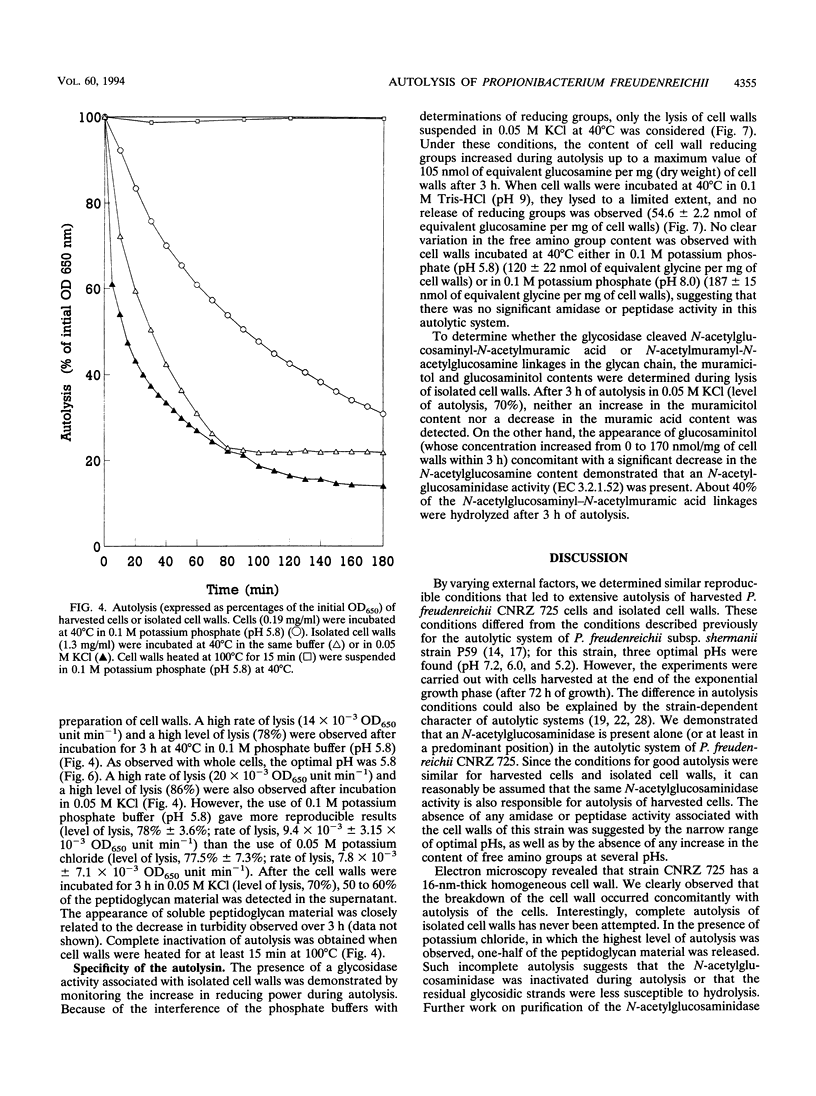
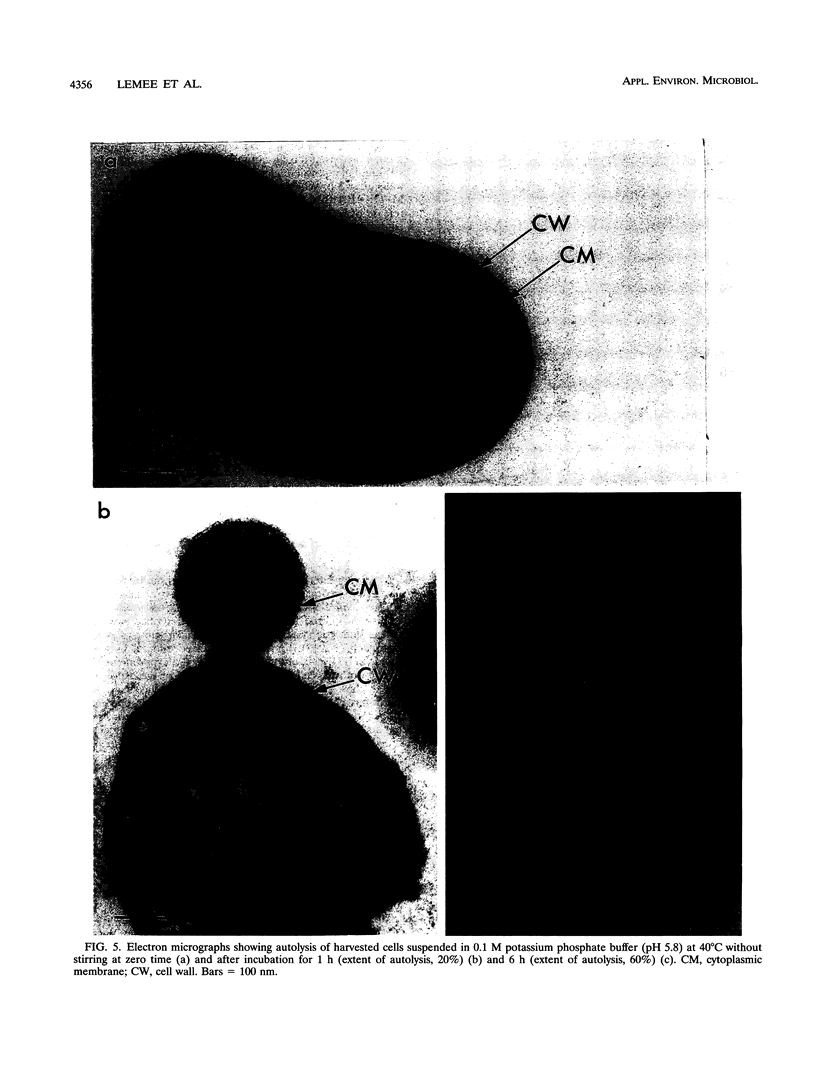
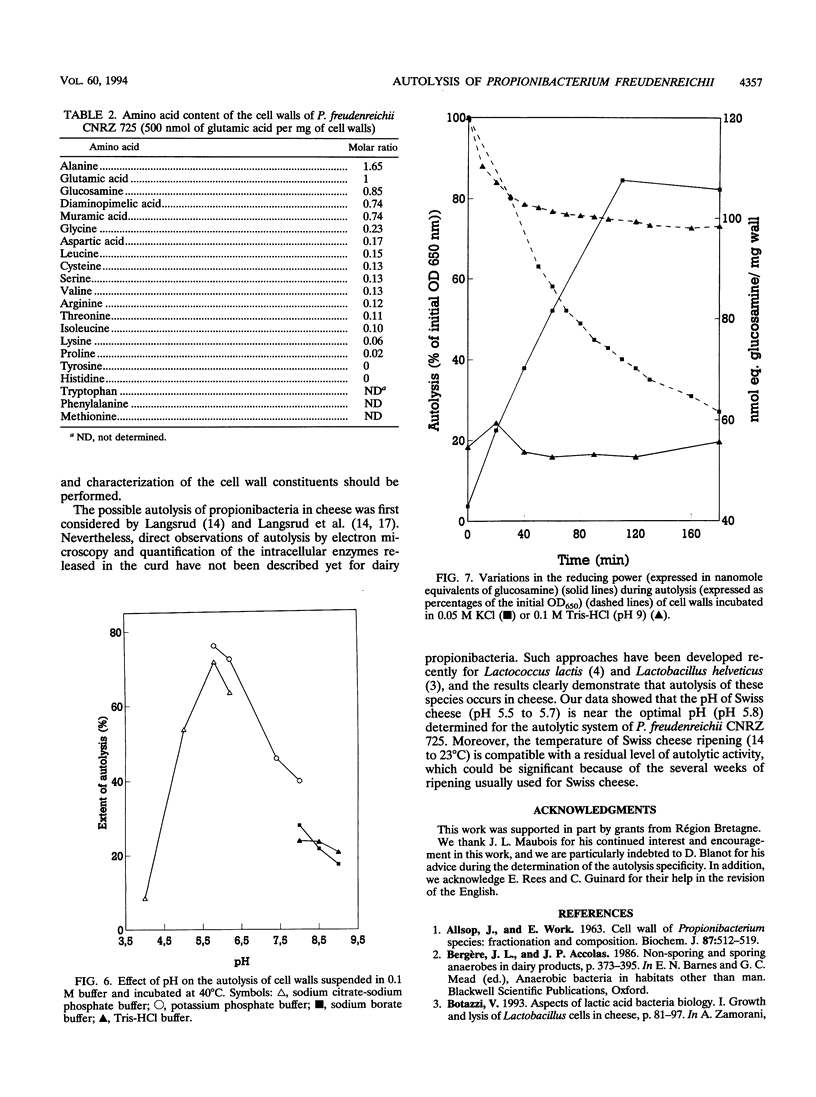
Propionibacterium freudenreichii plays an important role in Swiss cheese ripening (it produces propionic acid, acetic acid, and CO2). Moreover, autolysis of this organism certainly contributes to proteolysis and lipolysis of the curd because intracellular enzymes are released. By varying external factors, we determined the following conditions which promoted autolysis of both whole cells and isolated cell walls of P. freudenreichii CNRZ 725: (i) 0.1 M potassium phosphate buffer (pH 5.8) at 40°C and (ii) 0.05 to 0.1 M KCl at 40°C. We found that early-exponential-phase cells possessed the highest autolytic activity. It should be emphasized that the pH of Swiss cheese curd (pH 5.5 to 5.7) is near the optimal pH which we determined. Ultrastructural observations by electron microscopy revealed a 16-nm-thick homogeneous cell wall, as well as degradation of the cell wall that occurred concomitantly with cell autolysis. In the presence of 0.05 M potassium chloride, there was a great deal of isolated cell wall autolysis (the optical density at 650 nm decreased 77.5% ± 7.3% in 3 h), and one-half of the peptidoglycan material was released. Finally, the main autolytic activity was due to an N-acetylglucosaminidase activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLSOP J WORK E. Cell walls of Propionibacterium species: fractionation and composition. Biochem J. 1963 Jun;87:512–519. doi: 10.1042/bj0870512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergère J. L., Accolas J. P. Non-sporing and sporing anaerobes in dairy products. Soc Appl Bacteriol Symp Ser. 1986;13:373–396. [PubMed] [Google Scholar]
- Dupuis C., Corre C., Boyaval P. Lipase and Esterase Activities of Propionibacterium freudenreichii subsp. freudenreichii. Appl Environ Microbiol. 1993 Dec;59(12):4004–4009. doi: 10.1128/aem.59.12.4004-4009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisango K., Fujii H., Yanagihara Y., Mifuchi I., Azuma I. Structures of peptidoglycans of Propionibacterium. Microbiol Immunol. 1983;27(7):635–640. doi: 10.1111/j.1348-0421.1983.tb00625.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leduc M., van Heijenoort J. Autolysis of Escherichia coli. J Bacteriol. 1980 Apr;142(1):52–59. doi: 10.1128/jb.142.1.52-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A. C., Reinbold G. W., Vedamuthu E. R. An evaluation of the taxonomy of Propionibacterium. Can J Microbiol. 1968 Nov;14(11):1185–1191. doi: 10.1139/m68-199. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Plapp R., Kandler O. Glycine as crosslinking bridge in the LL-diaminopimelic acid containing murein of Propionibacterium peterssonii. FEBS Lett. 1968 Oct;1(5):287–290. doi: 10.1016/0014-5793(68)80133-4. [DOI] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak E., Kocoń J. Untersuchung der Zellstruktur von Propionsäurebakterien während ihrer Vermehrung. Zentralbl Mikrobiol. 1983;138(1):25–37. [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Williamson R., Ward J. B. Characterization of the autolytic enzymes of Clostridium perfringens. J Gen Microbiol. 1979 Oct;114(2):349–354. doi: 10.1099/00221287-114-2-349. [DOI] [PubMed] [Google Scholar]