Abstract
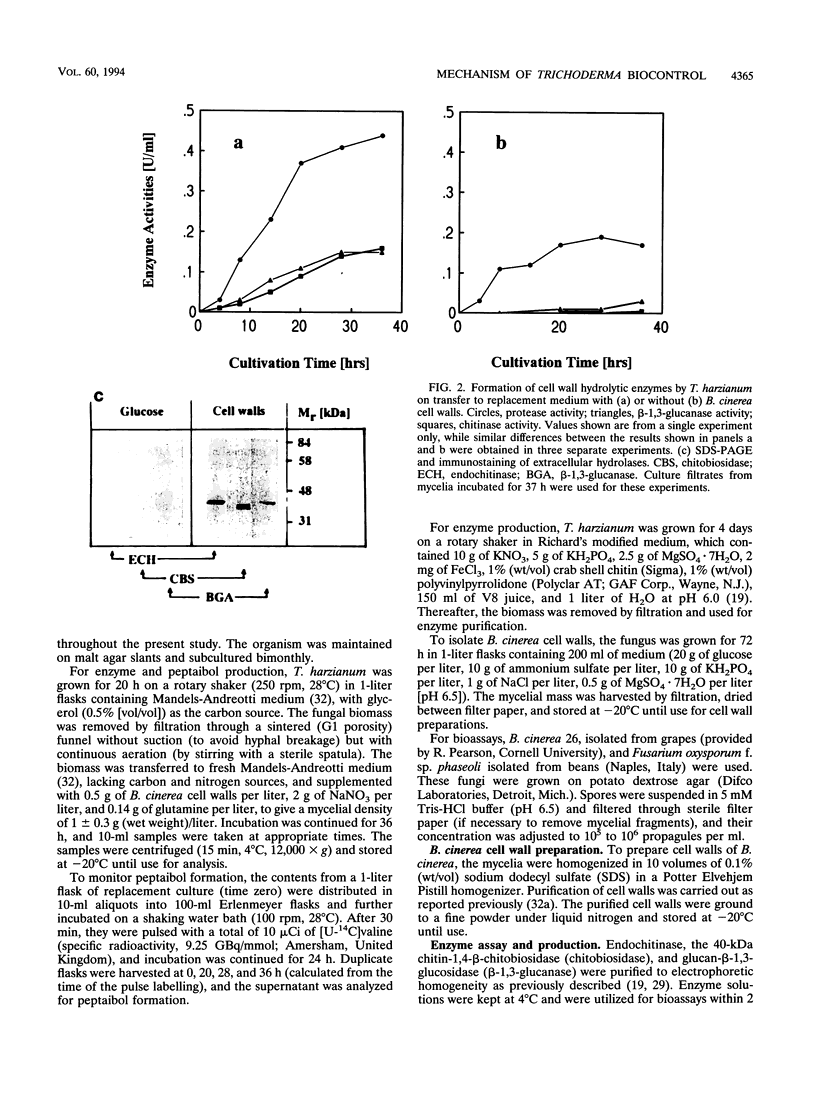
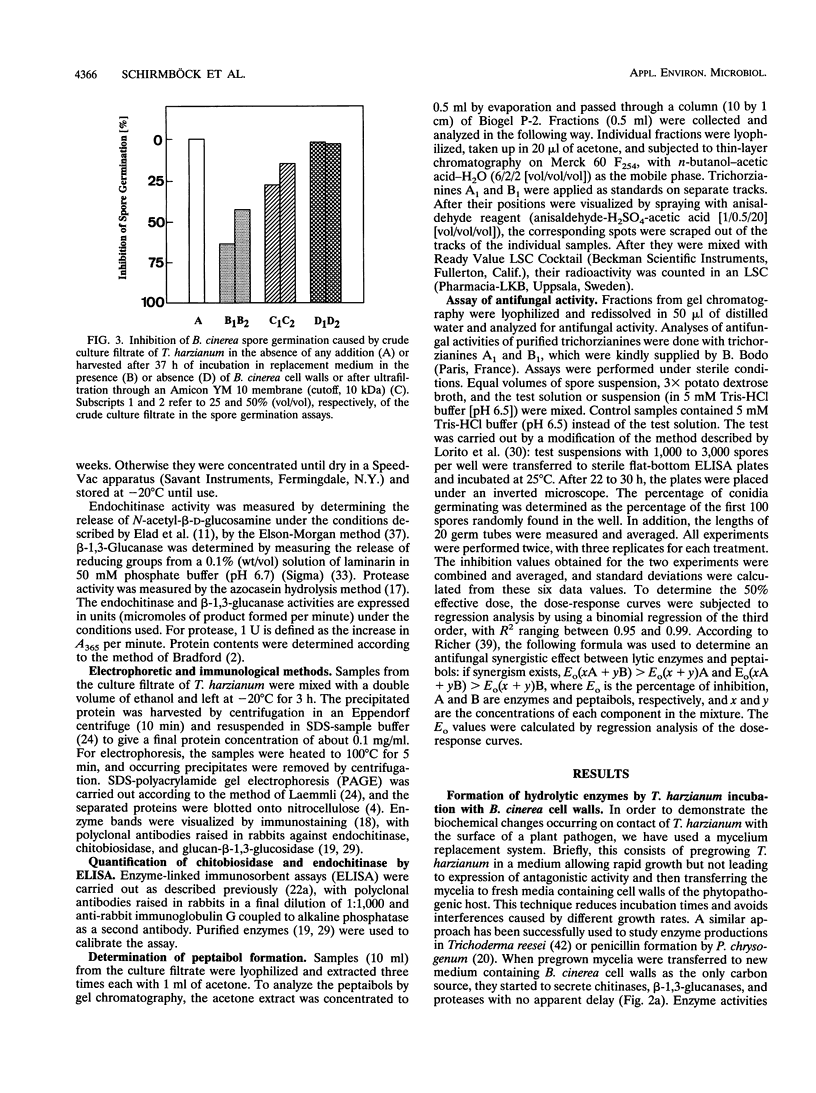
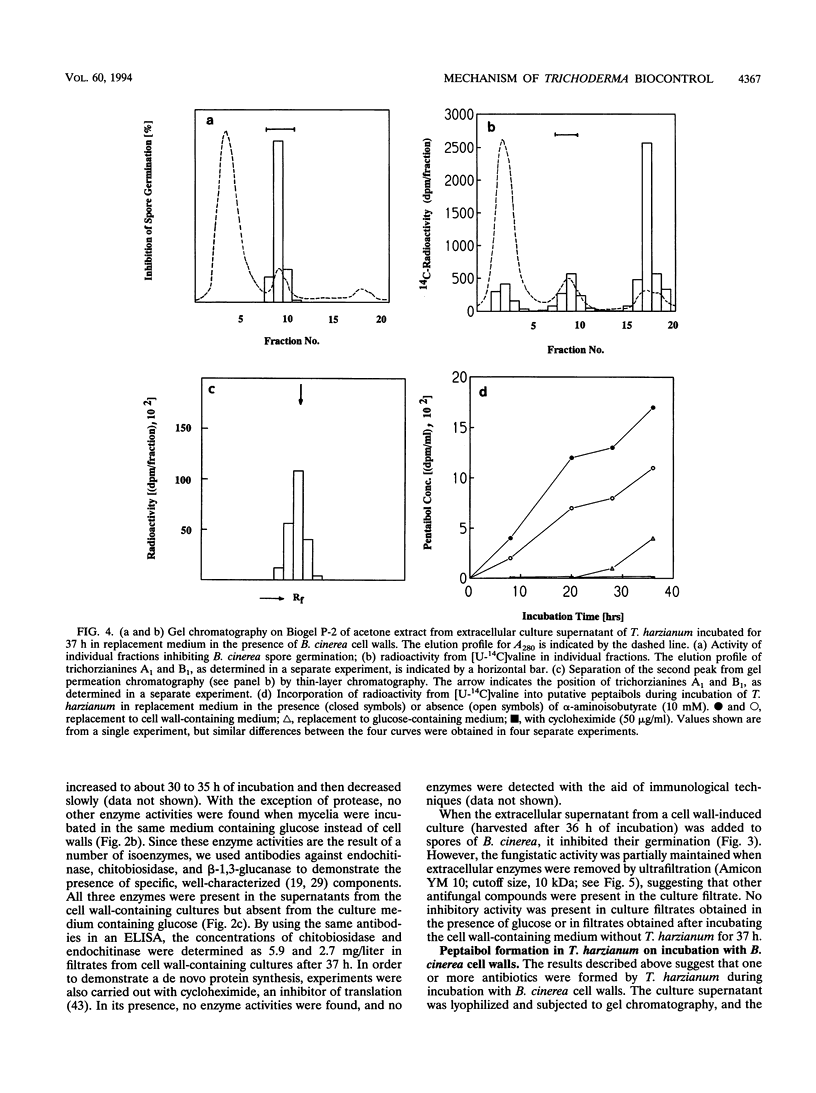
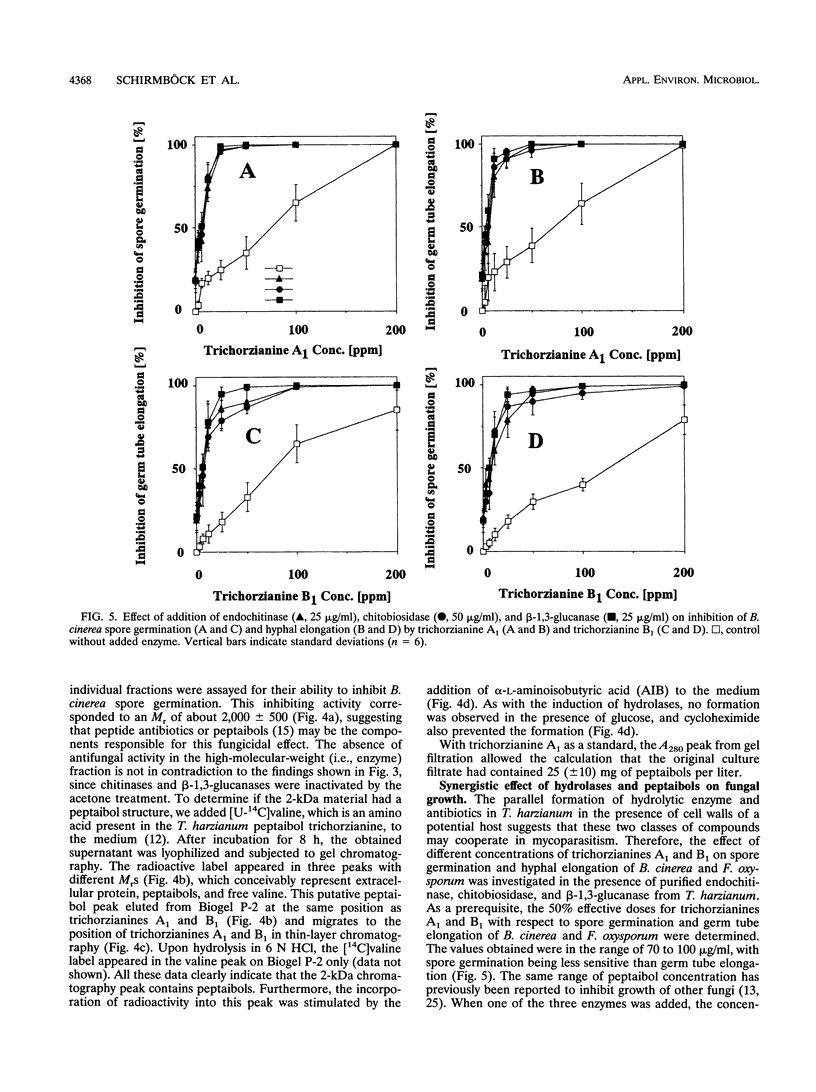
Chitinase, beta-1,3-glucanase, and protease activities were formed when Trichoderma harzianum mycelia, grown on glucose as the sole carbon source, were transferred to fresh medium containing cell walls of Botrytis cinerea. Chitobiohydrolase, endochitinase, and beta-1,3-glucanase activities were immunologically detected in culture supernatants by Western blotting (immunoblotting), and the first two were quantified by enzyme-linked immunosorbent assay. Under the same conditions, exogenously added [U-14C]valine was incorporated in acetone-soluble compounds with an apparent M(r) of < 2,000. These compounds comigrated with the peptaibols trichorzianines A1 and B1 in thin-layer chromatography and released [U-14C]valine after incubation in 6N HCl. Incorporation of radioactive valine into this material was stimulated by the exogenous supply of alpha-aminoisobutyric acid, a rare amino acid which is a major constituent of peptaibols. The obtained culture supernatants inhibited spore germination as well as hyphal elongation of B. cinerea. Culture supernatants from mycelia placed in fresh medium without cell walls of B. cinerea did not show hydrolase activities, incorporation of [U-14C]valine into peptaibol-like compounds, and inhibition of fungal growth. Purified trichorzianines A1 and B1 as well as purified chitobiohydrolase, endochitinase, or beta-1,3-glucanase inhibited spore germination and hyphal elongation, but at concentrations higher than those observed in the culture supernatants. However, when the enzymes and the peptaibols were tested together, an antifungal synergistic interaction was observed and the 50% effective dose values obtained were in the range of those determined in the culture supernatants. Therefore, the parallel formation and synergism of hydrolytic enzymes and antibiotics may have an important role in the antagonistic action of T. harzianum against fungal phytopathogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewer D., Mason F. G., Taylor A. The production of alamethicins by Trichoderma spp. Can J Microbiol. 1987 Jul;33(7):619–625. doi: 10.1139/m87-108. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Graeme-Cook K. A., Faull J. L. Effect of ultraviolet-induced mutants of Trichoderma harzianum with altered antibiotic production on selected pathogens in vitro. Can J Microbiol. 1991 Sep;37(9):659–664. doi: 10.1139/m91-112. [DOI] [PubMed] [Google Scholar]
- Hönlinger C., Kubicek C. P. Regulation of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine and isopenicillin N biosynthesis in Penicillium chrysogenum by the alpha-aminoadipate pool size. FEMS Microbiol Lett. 1989 Nov;53(1-2):71–75. doi: 10.1016/0378-1097(89)90368-6. [DOI] [PubMed] [Google Scholar]
- Inbar J., Chet I. Biomimics of fungal cell-cell recognition by use of lectin-coated nylon fibers. J Bacteriol. 1992 Feb;174(3):1055–1059. doi: 10.1128/jb.174.3.1055-1059.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe J., Kubicek C. P. Quantification and identification of the main components of the Trichoderma cellulase complex with monoclonal antibodies using an enzyme-linked immunosorbent assay (ELISA). Appl Microbiol Biotechnol. 1990 Oct;34(1):26–30. doi: 10.1007/BF00170918. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Doan T., el Hajji M., Rebuffat S., Rajesvari M. R., Bodo B. Fluorescence studies of the interaction of trichorzianine A IIIc with model membranes. Biochim Biophys Acta. 1986 Jun 13;858(1):1–5. doi: 10.1016/0005-2736(86)90284-1. [DOI] [PubMed] [Google Scholar]
- Lorito M., Peterbauer C., Hayes C. K., Harman G. E. Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology. 1994 Mar;140(Pt 3):623–629. doi: 10.1099/00221287-140-3-623. [DOI] [PubMed] [Google Scholar]
- Molle G., Duclohier H., Spach G. Voltage-dependent and multi-state ionic channels induced by trichorzianines, anti-fungal peptides related to alamethicin. FEBS Lett. 1987 Nov 16;224(1):208–212. doi: 10.1016/0014-5793(87)80449-0. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Reusser F. Biosynthesis of antibiotic U-22,324, a cyclic polypeptide. J Biol Chem. 1967 Jan 25;242(2):243–247. [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979 Sep;139(3):761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Hajji M., Rebuffat S., Le Doan T., Klein G., Satre M., Bodo B. Interaction of trichorzianines A and B with model membranes and with the amoeba Dictyostelium. Biochim Biophys Acta. 1989 Jan 16;978(1):97–104. doi: 10.1016/0005-2736(89)90504-x. [DOI] [PubMed] [Google Scholar]
- el Hajji M., Rebuffat S., Lecommandeur D., Bodo B. Isolation and sequence determination of trichorzianines A antifungal peptides from Trichoderma harzianum. Int J Pept Protein Res. 1987 Feb;29(2):207–215. doi: 10.1111/j.1399-3011.1987.tb02247.x. [DOI] [PubMed] [Google Scholar]





