Abstract
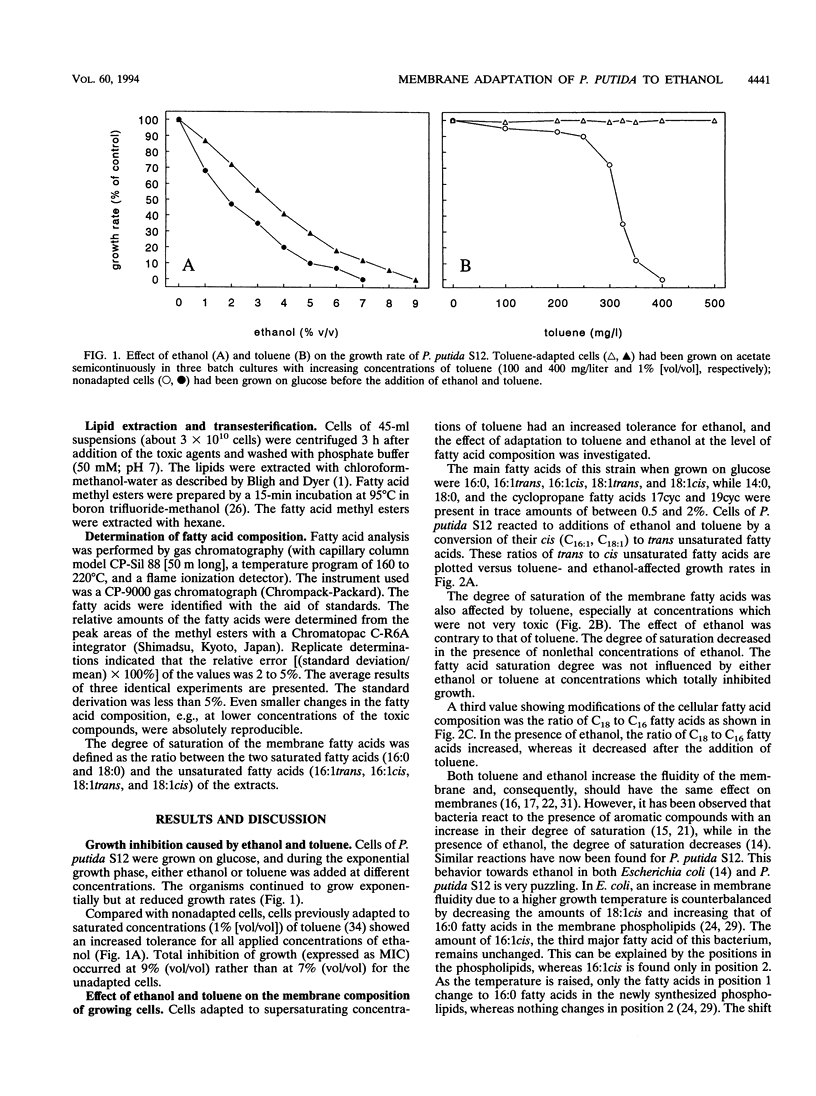
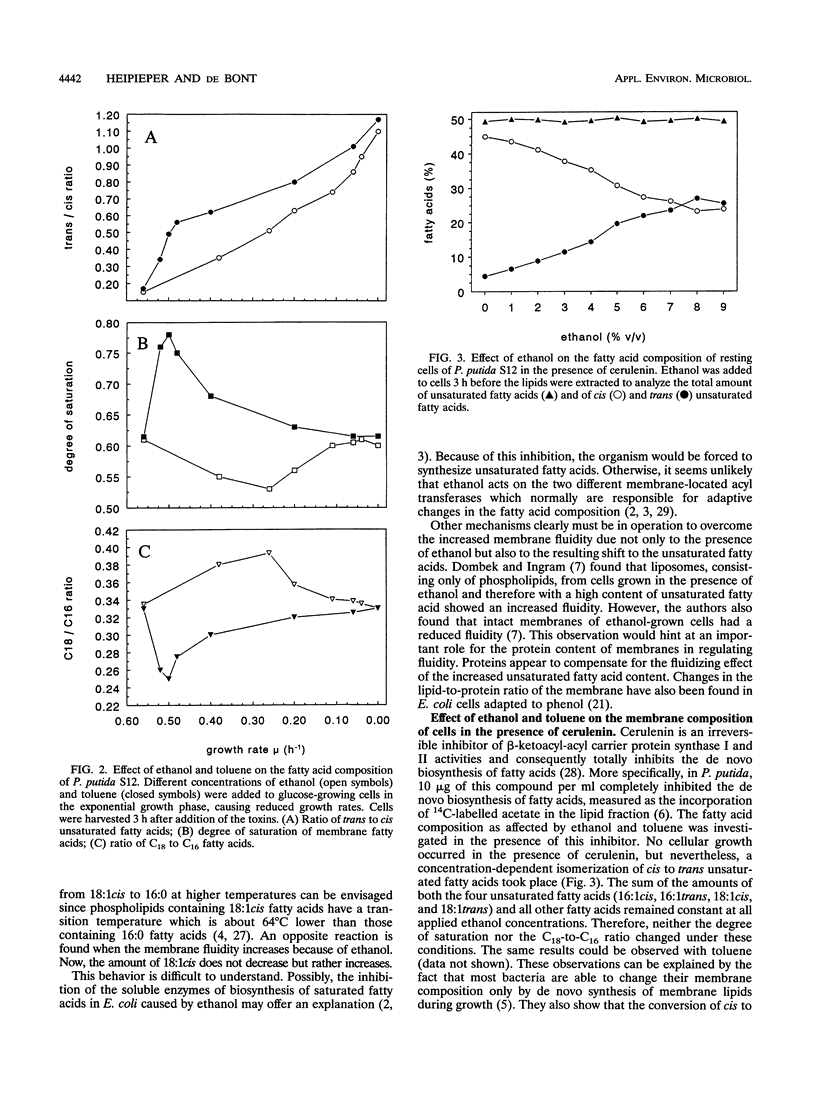
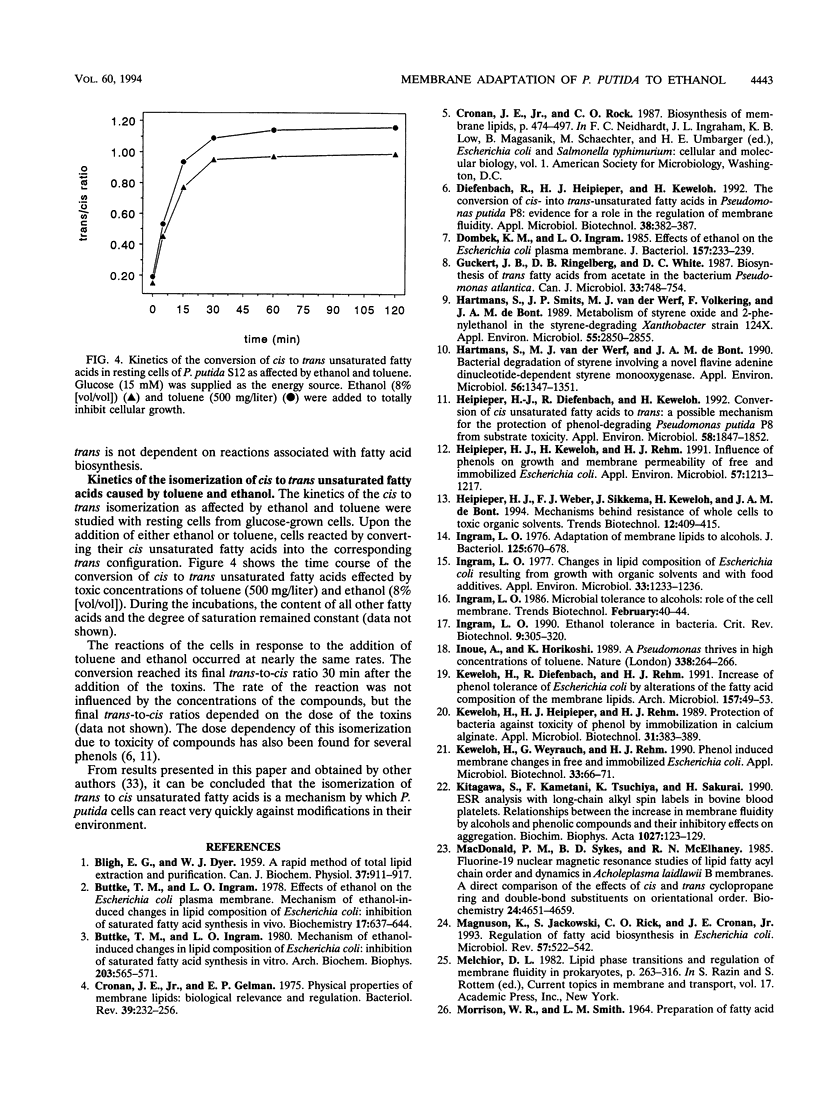
Pseudomonas putida S12 was more tolerant to ethanol when preadapted to supersaturating concentrations of toluene. Cellular reactions at the membrane level to the toxicities of both compounds were different. In growing cells of P. putida S12, sublethal concentrations of toluene resulted in an increase in the degree of saturation of the membrane fatty acids, whereas toxically equivalent concentrations of ethanol led to a decrease in this value. Contrary to this, cells also reacted to both substances with a strong increase of the trans unsaturated fatty acids and a corresponding decrease of the cis unsaturated fatty acids under conditions where growth and other cellular membrane reactions were totally inhibited. While the isomerization of cis to trans unsaturated fatty acids compensates for the fluidizing effect caused by ethanol, a decrease in the degree of saturation is antagonistic with respect to the chemo-physical properties of the membrane. Consequently, the results support the hypothesis that the decrease in the degree of saturation induced by ethanol is not an adaptation mechanism but is caused by an inhibitory effect of the compound on the biosynthesis of saturated fatty acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vitro. Arch Biochem Biophys. 1980 Sep;203(2):565–571. doi: 10.1016/0003-9861(80)90213-1. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Mechanism of ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vivo. Biochemistry. 1978 Feb 21;17(4):637–644. doi: 10.1021/bi00597a012. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O. Effects of ethanol on the Escherichia coli plasma membrane. J Bacteriol. 1984 Jan;157(1):233–239. doi: 10.1128/jb.157.1.233-239.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmans S., Smits J. P., van der Werf M. J., Volkering F., de Bont J. A. Metabolism of Styrene Oxide and 2-Phenylethanol in the Styrene-Degrading Xanthobacter Strain 124X. Appl Environ Microbiol. 1989 Nov;55(11):2850–2855. doi: 10.1128/aem.55.11.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmans S., van der Werf M. J., de Bont J. A. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol. 1990 May;56(5):1347–1351. doi: 10.1128/aem.56.5.1347-1351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heipieper H. J., Diefenbach R., Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992 Jun;58(6):1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heipieper H. J., Keweloh H., Rehm H. J. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol. 1991 Apr;57(4):1213–1217. doi: 10.1128/aem.57.4.1213-1217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Changes in lipid composition of Escherichia coli resulting from growth with organic solvents and with food additives. Appl Environ Microbiol. 1977 May;33(5):1233–1236. doi: 10.1128/aem.33.5.1233-1236.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Ethanol tolerance in bacteria. Crit Rev Biotechnol. 1990;9(4):305–319. doi: 10.3109/07388558909036741. [DOI] [PubMed] [Google Scholar]
- Keweloh H., Diefenbach R., Rehm H. J. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol. 1991;157(1):49–53. doi: 10.1007/BF00245334. [DOI] [PubMed] [Google Scholar]
- Keweloh H., Weyrauch G., Rehm H. J. Phenol-induced membrane changes in free and immobilized Escherichia coli. Appl Microbiol Biotechnol. 1990 Apr;33(1):66–71. doi: 10.1007/BF00170572. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Kametani F., Tsuchiya K., Sakurai H. ESR analysis with long-chain alkyl spin labels in bovine blood platelets. Relationship between the increase in membrane fluidity by alcohols and phenolic compounds and their inhibitory effects on aggregation. Biochim Biophys Acta. 1990 Aug 24;1027(2):123–129. doi: 10.1016/0005-2736(90)90075-y. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Macdonald P. M., Sykes B. D., McElhaney R. N. Fluorine-19 nuclear magnetic resonance studies of lipid fatty acyl chain order and dynamics in Acholeplasma laidlawii B membranes. A direct comparison of the effects of cis and trans cyclopropane ring and double-bond substituents on orientational order. Biochemistry. 1985 Aug 13;24(17):4651–4659. doi: 10.1021/bi00338a026. [DOI] [PubMed] [Google Scholar]
- Magnuson K., Jackowski S., Rock C. O., Cronan J. E., Jr Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993 Sep;57(3):522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H., Okajima N., Sasaki S., Higashi S., Murata N. The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochim Biophys Acta. 1991 Jun 19;1084(1):13–20. doi: 10.1016/0005-2760(91)90049-n. [DOI] [PubMed] [Google Scholar]
- Omura S. Cerulenin. Methods Enzymol. 1981;72:520–532. [PubMed] [Google Scholar]
- Rock C. O. Turnover of fatty acids in the 1-position of phosphatidylethanolamine in Escherichia coli. J Biol Chem. 1984 May 25;259(10):6188–6194. [PubMed] [Google Scholar]
- Sikkema J., Poolman B., Konings W. N., de Bont J. A. Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J Bacteriol. 1992 May;174(9):2986–2992. doi: 10.1128/jb.174.9.2986-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema J., de Bont J. A., Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994 Mar 18;269(11):8022–8028. [PubMed] [Google Scholar]
- Weber F. J., Isken S., de Bont J. A. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994 Aug;140(Pt 8):2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]
- Weber F. J., Ooijkaas L. P., Schemen R. M., Hartmans S., de Bont J. A. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl Environ Microbiol. 1993 Oct;59(10):3502–3504. doi: 10.1128/aem.59.10.3502-3504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



