Abstract
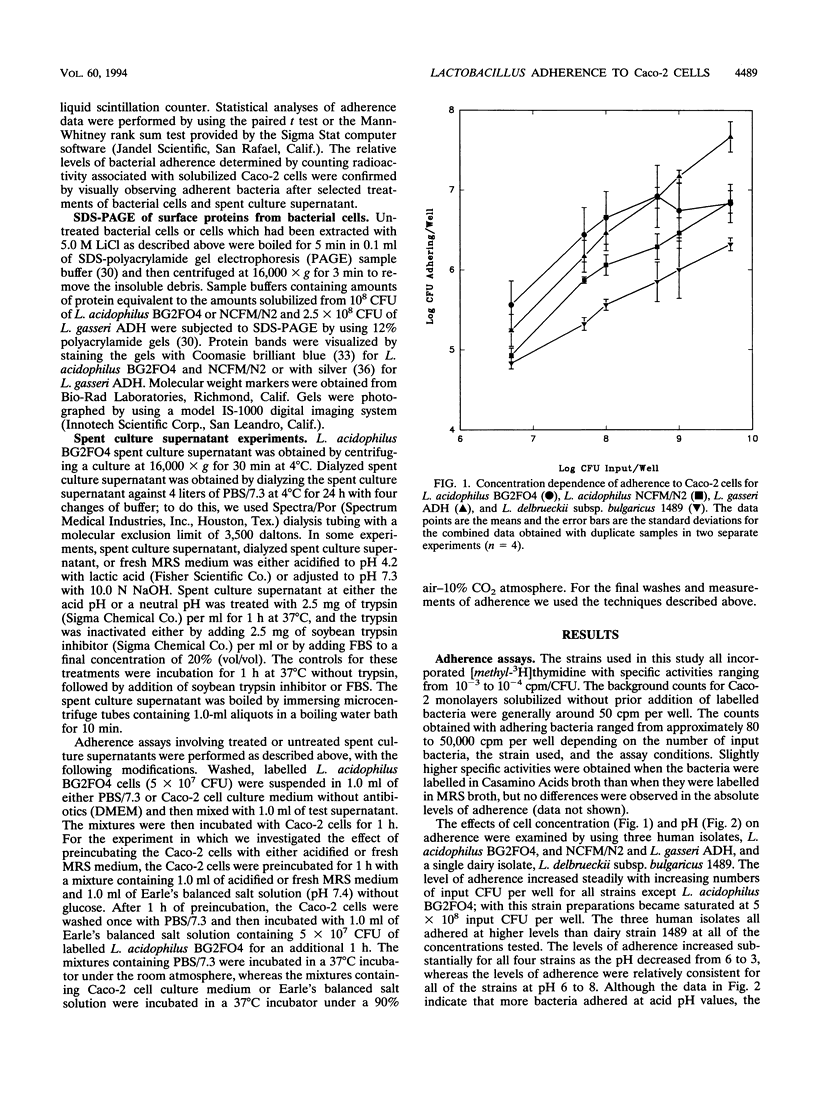
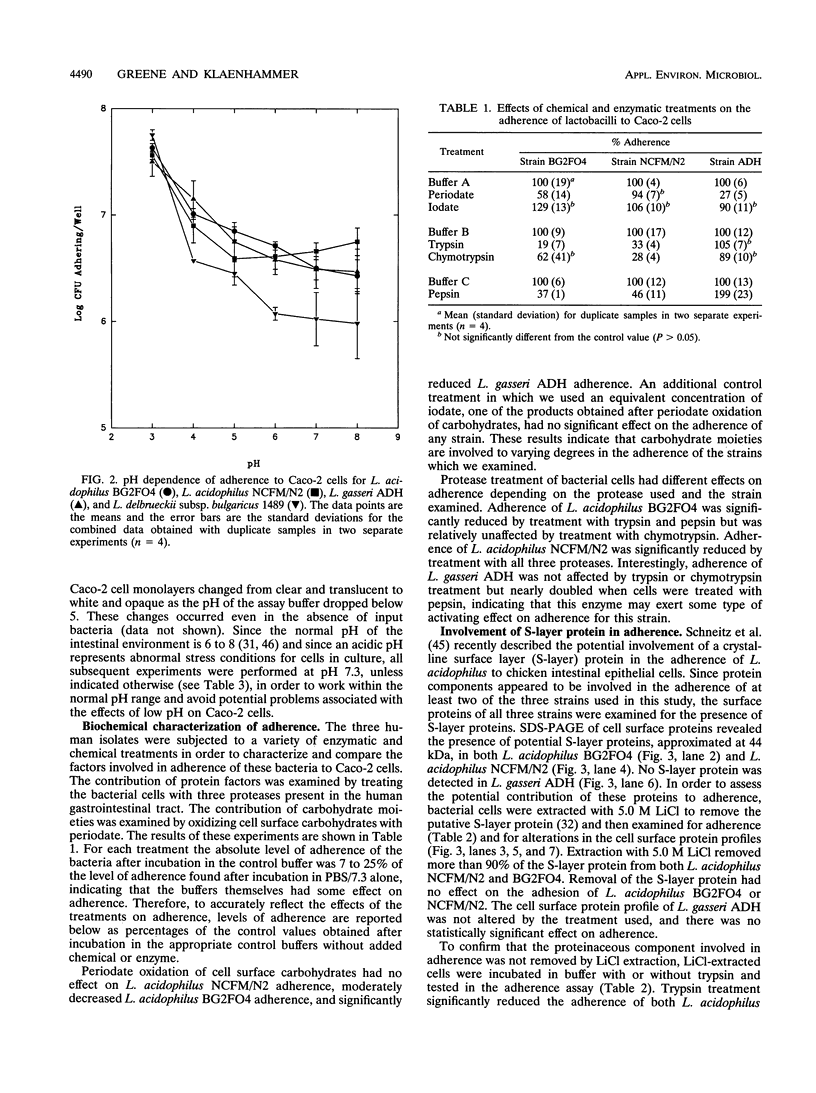
A quantitative assay performed with bacterial cells labelled with [3H]thymidine was used to investigate factors involved in the adherence of human isolates Lactobacillus acidophilus BG2FO4 and NCFM/N2 and Lactobacillus gasseri ADH to human Caco-2 intestinal cells. For all three strains, adherence was concentration dependent, greater at acidic pH values, and significantly greater than adherence of a control dairy isolate, Lactobacillus delbrueckii subsp. bulgaricus 1489. Adherence of L. acidophilus BG2FO4 and NCFM/N2 was decreased by protease treatment of the bacterial cells, whereas adherence of L. gasseri ADH either was not affected or was enhanced by protease treatment. Putative surface layer proteins were identified on L. acidophilus BG2FO4 and NCFM/N2 cells but were not involved in adherence. Periodate oxidation of bacterial cell surface carbohydrates significantly reduced adherence of L. gasseri ADH, moderately reduced adherence of L. acidophilus BG2FO4, and had no effect on adherence of L. acidophilus NCFM/N2. These results indicate that Lactobacillus species adhere to human intestinal cells via mechanisms which involve different combinations of carbohydrate and protein factors on the bacterial cell surface. The involvement of a secreted bridging protein, which has been proposed as the primary mediator of adherence of L. acidophilus BG2FO4 in spent culture supernatant (M.-H. Coconnier, T. R. Klaenhammer, S. Kernéis, M.-F. Bernet, and A. L. Servin, Appl. Environ. Microbiol. 58:2034-2039, 1992), was not confirmed in this study. Rather, a pH effect on Caco-2 cells contributed significantly to the adherence of this strain in spent culture supernatant.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barefoot S. F., Klaenhammer T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983 Jun;45(6):1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet M. F., Brassart D., Neeser J. R., Servin A. L. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol. 1993 Dec;59(12):4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot H. J., Kolen C. P., van Noort J. M., Pouwels P. H. S-layer protein of Lactobacillus acidophilus ATCC 4356: purification, expression in Escherichia coli, and nucleotide sequence of the corresponding gene. J Bacteriol. 1993 Oct;175(19):6089–6096. doi: 10.1128/jb.175.19.6089-6096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker B. E., Fuller R. Adhesion of Lactobacilli to the chicken crop epithelium. J Ultrastruct Res. 1975 Jul;52(1):21–31. doi: 10.1016/s0022-5320(75)80019-0. [DOI] [PubMed] [Google Scholar]
- Chan R. C., Reid G., Irvin R. T., Bruce A. W., Costerton J. W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985 Jan;47(1):84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvière G., Coconnier M. H., Kerneis S., Darfeuille-Michaud A., Joly B., Servin A. L. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol Lett. 1992 Mar 15;70(3):213–217. doi: 10.1016/0378-1097(92)90700-x. [DOI] [PubMed] [Google Scholar]
- Chauvière G., Coconnier M. H., Kernéis S., Fourniat J., Servin A. L. Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol. 1992 Aug;138(Pt 8):1689–1696. doi: 10.1099/00221287-138-8-1689. [DOI] [PubMed] [Google Scholar]
- Coconnier M. H., Klaenhammer T. R., Kernéis S., Bernet M. F., Servin A. L. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl Environ Microbiol. 1992 Jun;58(6):2034–2039. doi: 10.1128/aem.58.6.2034-2039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway P. L., Gorbach S. L., Goldin B. R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987 Jan;70(1):1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Kjelleberg S. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J Gen Microbiol. 1989 May;135(5):1175–1186. doi: 10.1099/00221287-135-5-1175. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Aubel D., Chauviere G., Rich C., Bourges M., Servin A., Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990 Apr;58(4):893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990 Nov;162(5):1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Heffron F., Finlay B. B., Falkow S. Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun. 1990 Feb;58(2):443–448. doi: 10.1128/iai.58.2.443-448.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Sailer M., Johnson K., Roy K. L., Vederas J. C., Stiles M. E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991 Dec;173(23):7491–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson A., Conway P. L. Adhesion to porcine squamous epithelium of saccharide and protein moieties of Lactobacillus fermentum strain 104-S. J Gen Microbiol. 1992 Dec;138(12):2657–2661. doi: 10.1099/00221287-138-12-2657. [DOI] [PubMed] [Google Scholar]
- Henriksson A., Szewzyk R., Conway P. L. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl Environ Microbiol. 1991 Feb;57(2):499–502. doi: 10.1128/aem.57.2.499-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeman E. G., Klaenhammer T. R. Adherence of Lactobacillus species to human fetal intestinal cells. J Dairy Sci. 1982 Nov;65(11):2063–2069. doi: 10.3168/jds.S0022-0302(82)82462-4. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Savage D. C. Models for study of the specificity by which indigenous lactobacilli adhere to murine gastric epithelia. Infect Immun. 1979 Dec;26(3):966–975. doi: 10.1128/iai.26.3.966-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCarthy D. M., Lin J. H., Rinckel L. A., Savage D. C. Genetic transformation in Lactobacillus sp. strain 100-33 of the capacity to colonize the nonsecreting gastric epithelium in mice. Appl Environ Microbiol. 1988 Feb;54(2):416–422. doi: 10.1128/aem.54.2.416-422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin G., Jeppsson B., Johansson M. L., Ahrné S., Nobaek S., Ståhl M., Bengmark S. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J Appl Bacteriol. 1993 Mar;74(3):314–323. doi: 10.1111/j.1365-2672.1993.tb03031.x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi P., Tall B. D., Russell R. G., Detolla L. J., Morris J. G., Jr Development of an in vitro model for study of non-O1 Vibrio cholerae virulence using Caco-2 cells. Infect Immun. 1990 Oct;58(10):3415–3424. doi: 10.1128/iai.58.10.3415-3424.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya R. R., Fremaux C., De Antoni G. L., Klaenhammer T. R. Site-specific integration of the temperate bacteriophage phi adh into the Lactobacillus gasseri chromosome and molecular characterization of the phage (attP) and bacterial (attB) attachment sites. J Bacteriol. 1992 Sep;174(17):5584–5592. doi: 10.1128/jb.174.17.5584-5592.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya R. R., Kleeman E. G., Luchansky J. B., Klaenhammer T. R. Characterization of the temperate bacteriophage phi adh and plasmid transduction in Lactobacillus acidophilus ADH. Appl Environ Microbiol. 1989 Sep;55(9):2206–2213. doi: 10.1128/aem.55.9.2206-2213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Servin A. L., Bruce A. W., Busscher H. J. Adhesion of three Lactobacillus strains to human urinary and intestinal epithelial cells. Microbios. 1993;75(302):57–65. [PubMed] [Google Scholar]
- Sanders M. E. Effect of consumption of lactic cultures on human health. Adv Food Nutr Res. 1993;37:67–130. doi: 10.1016/s1043-4526(08)60116-3. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Schneitz C., Nuotio L., Lounatma K. Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer). J Appl Bacteriol. 1993 Mar;74(3):290–294. doi: 10.1111/j.1365-2672.1993.tb03028.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Tannock G. W. The normal microflora: new concepts in health promotion. Microbiol Sci. 1988 Jan;5(1):4–8. [PubMed] [Google Scholar]
- Vidgrén G., Palva I., Pakkanen R., Lounatmaa K., Palva A. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of the nucleotide sequence. J Bacteriol. 1992 Nov;174(22):7419–7427. doi: 10.1128/jb.174.22.7419-7427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Andersson K., Sydow M., Axelsson L., Lindgren S., Gullmar B. Surface properties of lactobacilli isolated from the small intestine of pigs. J Appl Bacteriol. 1987 Jun;62(6):513–520. doi: 10.1111/j.1365-2672.1987.tb02683.x. [DOI] [PubMed] [Google Scholar]