Abstract
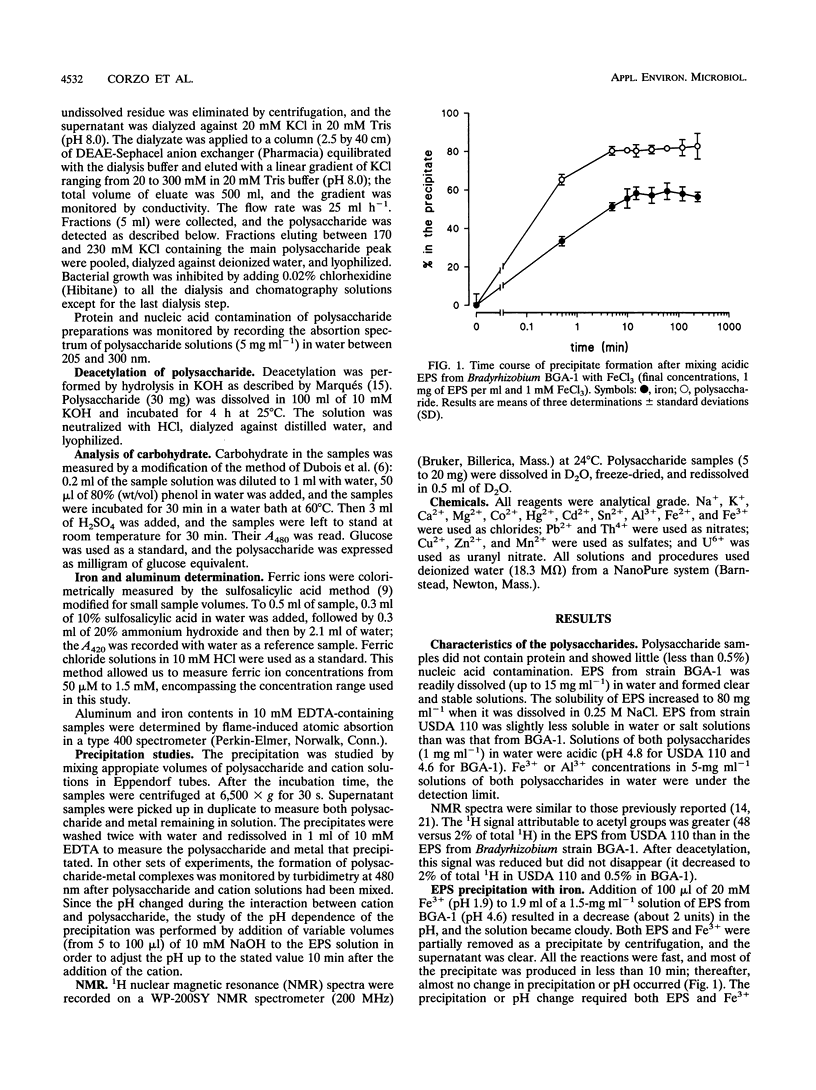
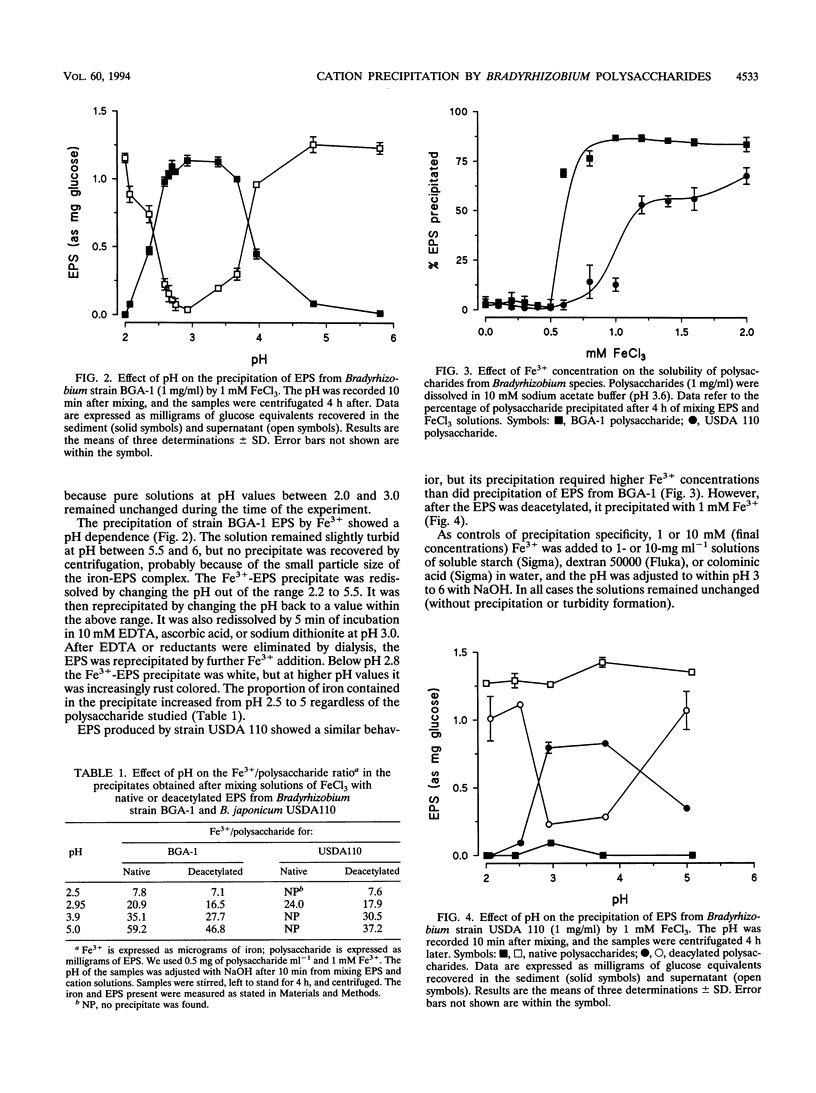
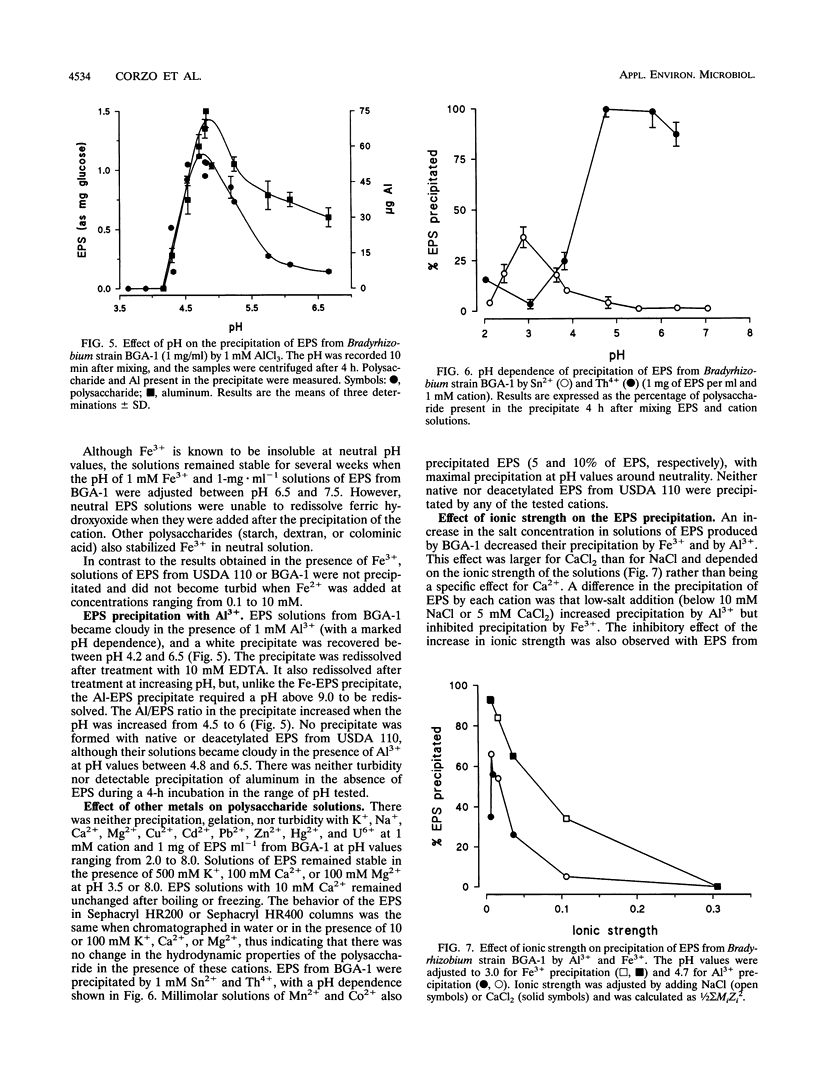
The interaction between the acidic exopolysaccharides produced by two Bradyrhizobium strains and several metal cations has been studied. Aqueous solutions in the millimolar range of Fe3+ but not of Fe2+ precipitated the exopolysaccharides from Bradyrhizobium (Chamaecytisus) strain BGA-1 and, to a lesser extent, Bradyrhizobium japonicum USDA 110. The precipitation was pH dependent, with a maximum around pH 3. The precipitate was redissolved by changing the pH and by Fe3+ reduction or chelation. Deacetylation of B. japonicum polysaccharide increased its precipitation by Fe3+. At pH near neutrality, the polysaccharide from Bradyrhizobium (Chamaecytisus) strain BGA-1 stabilized Fe3+ solutions, despite the insolubility of Fe(OH)3. Aluminum precipitated Bradyrhizobium (Chamaecytisus) polysaccharide but not the polysaccharide produced by B. japonicum. The precipitation showed a maximum at about pH 4.8, and the precipitate was redissolved after Al3+ chelation with EDTA. Precipitation was inhibited by increases in the ionic strength over 10 mM. Bradyrhizobium (Chamaecytisus) polysaccharide was also precipitated by Th4+, Sn2+, Mn2+, and Co2+. The presence of Fe3+ increased the exopolysaccharide precipitation by aluminum. No precipitation, gelation, or increase in turbidity of polysaccharide solutions occurred when K+, Na+, Ca2+, Mg2+, Cu2+, Cd2+, Pb2+, Zn2+, Hg2+, or U6+ was added at several pH values. The results suggest that the precipitation is based on the interaction between carboxylate groups from different polysaccharide chains and the partially hydrolyzed aquoions of Fe3+, Al3+, Th4+, and Sn2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J. Role of cellular design in bacterial metal accumulation and mineralization. Annu Rev Microbiol. 1989;43:147–171. doi: 10.1146/annurev.mi.43.100189.001051. [DOI] [PubMed] [Google Scholar]
- Ghiorse W. C. Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol. 1984;38:515–550. doi: 10.1146/annurev.mi.38.100184.002503. [DOI] [PubMed] [Google Scholar]
- McLean R. J., Beauchemin D., Beveridge T. J. Influence of oxidation state on iron binding by Bacillus licheniformis capsule. Appl Environ Microbiol. 1992 Jan;58(1):405–408. doi: 10.1128/aem.58.1.405-408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. J., Beauchemin D., Clapham L., Beveridge T. J. Metal-Binding Characteristics of the Gamma-Glutamyl Capsular Polymer of Bacillus licheniformis ATCC 9945. Appl Environ Microbiol. 1990 Dec;56(12):3671–3677. doi: 10.1128/aem.56.12.3671-3677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman M. W., Geesey G. G. Copper-binding characteristics of exopolymers from a freshwater-sediment bacterium. Appl Environ Microbiol. 1985 Apr;49(4):846–851. doi: 10.1128/aem.49.4.846-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. R., Rees D. A., Thom D., Welsh E. J. Conformation and intermolecular interactions of carbohydrate chains. J Supramol Struct. 1977;6(2):259–274. doi: 10.1002/jss.400060211. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Application of two new methods for cleavage of polysaccharides into specific oligosaccharide fragments. Structure of the capsular and extracellular polysaccharides of Rhizobium japonicum that bind soybean lectin. J Biol Chem. 1982 Feb 25;257(4):1870–1875. [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plude John L., Parker Dorothy L., Schommer Olivia J., Timmerman Robert J., Hagstrom Stephanie A., Joers James M., Hnasko Robert. Chemical Characterization of Polysaccharide from the Slime Layer of the Cyanobacterium Microcystis flos-aquae C3-40. Appl Environ Microbiol. 1991 Jun;57(6):1696–1700. doi: 10.1128/aem.57.6.1696-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


